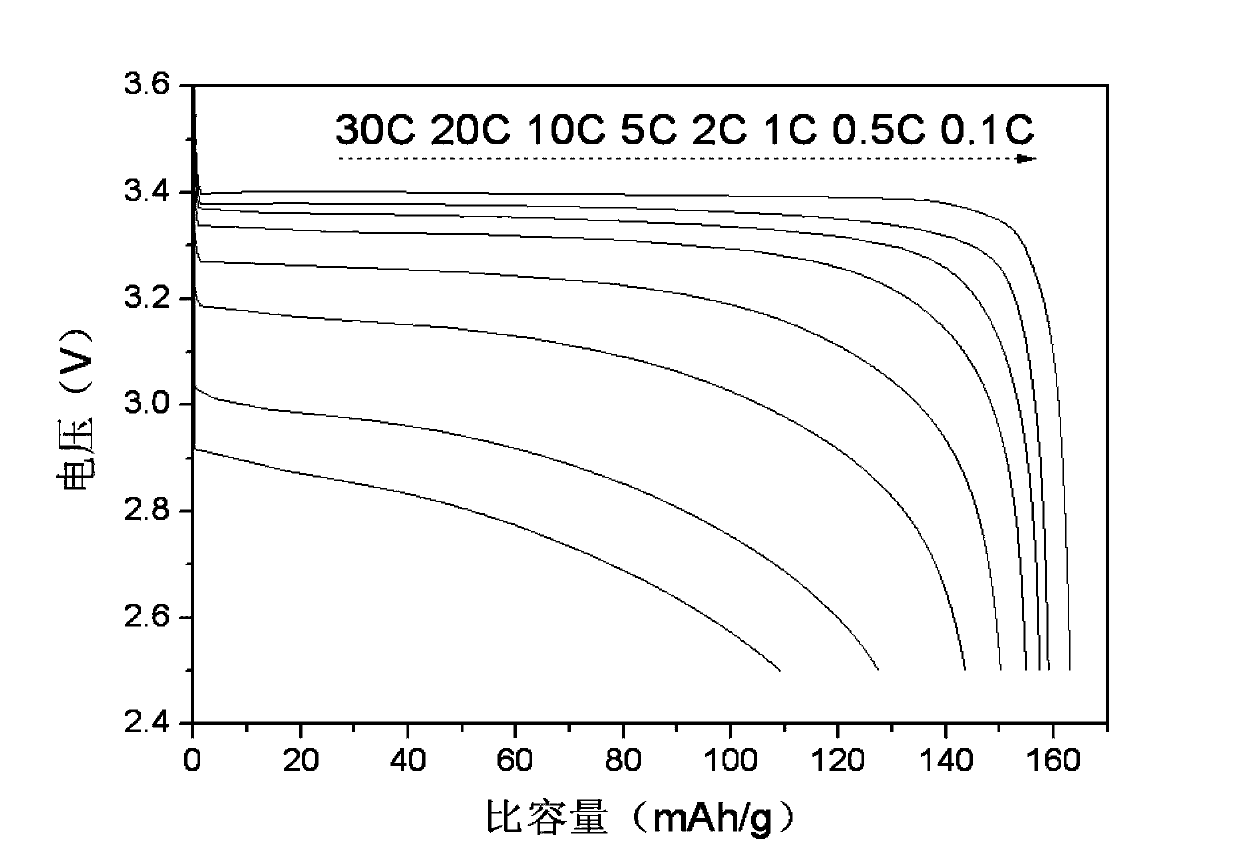
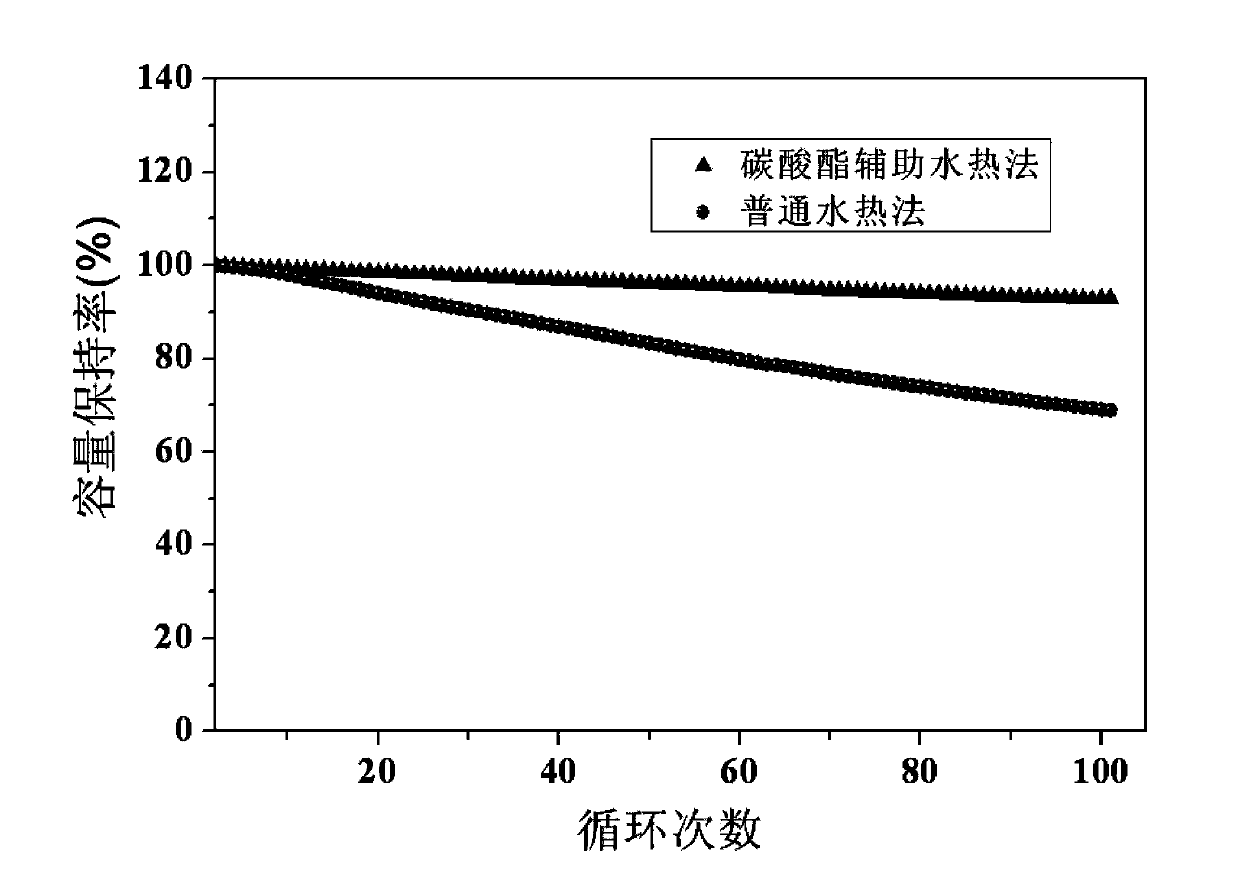
Carbonate-assisted preparation method for lithium iron phosphate
A carbonate and lithium source technology, applied in the field of hydrothermal preparation of lithium iron phosphate cathode materials, can solve the problems of poor high rate performance, poor consistency, poor cycle performance, etc., to improve cycle stability, batch Good stability and improved cycle performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Take LiOH·H 2 O, H 3 PO 4 , FeSO 4 ·7H 2 O is the basic raw material, and according to the molar ratio Li:Fe:P=3.0~3.05:1:1~1.05, it is made into an aqueous solution and mixed and stirred evenly. The concentration of the reactant is 0.5mol / L in terms of lithium ion concentration. 2 SO 4 Adjust the pH value of the reactant to 8. In order to prevent the ferrous iron from being oxidized, the whole stirring process is carried out under the protection of Ar, and then the volume ratio is 50% ethylene carbonate (EC) and the above solution are fully mixed and stirred for 30 minutes, and then quickly moved into 500ml React at 180°C for 600 minutes in a hydrothermal reactor.
[0041] After the above reaction is completed, the resulting precipitate is washed with deionized water and ethanol until the pH of the solution is neutral, and the obtained precipitate is dried under vacuum at 100°C for 5 hours to obtain off-white LiFePO 4 powder. The above gray-white LiFePO 4 After...
Embodiment 2
[0043] Take LiOH·H 2 O, H 3 PO 4 , FeCl 2 As the basic raw material, according to the molar ratio of Li:Fe:P=3.0~3.05:1:1~1.05, it is made into an aqueous solution and mixed and stirred evenly. The concentration of the reactant is 1mol / L in terms of lithium ion concentration, and the pH of the reactant is adjusted by HCl The value is 10. In order to prevent the ferrous iron from being oxidized, Ar protective gas is introduced during the whole stirring process, and then the volume ratio is 20% propylene carbonate (PC) and the above solution are fully mixed and stirred for 30 minutes, and then quickly moved into 500ml of hydrothermal The reaction was carried out at 180° C. for 600 minutes in the reaction kettle.
[0044] After the above reaction is completed, the precipitate is washed with a mixture of deionized water and ethanol until the pH of the solution is neutral, and the obtained precipitate is vacuum-dried at 120°C for 5 hours to obtain off-white LiFePO 4 powder. Th...
Embodiment 3
[0046] With Li 2 CO 3 、H 3 PO 4 、Fe(Ac) 2 ) 2 As the basic raw material, according to the molar ratio of Li:Fe:P=3.0~3.05:1:1~1.05, it is made into an aqueous solution and mixed and stirred evenly. The concentration of the reactant is 0.5mol / L in terms of lithium ion concentration. 3 PO 4 Regulate reactant pH value to be 9, in order to prevent ferrous iron from being oxidized, pass into Ar protective gas during the whole stirring process, be that 50% diethyl carbonate (DEC) is mixed with above-mentioned solution fully then and stir quickly after 30 minutes by volume ratio Transfer to a 500ml hydrothermal reactor and react at 200°C for 600 minutes.
[0047] After the above reaction is completed, the product is washed with deionized water and ethanol until the pH of the solution is neutral, and the obtained precipitate is vacuum-dried at 120°C for 5 hours to obtain off-white LiFePO 4 powder. The above gray-white LiFePO 4 The powder product and ascorbic acid are fully mi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com