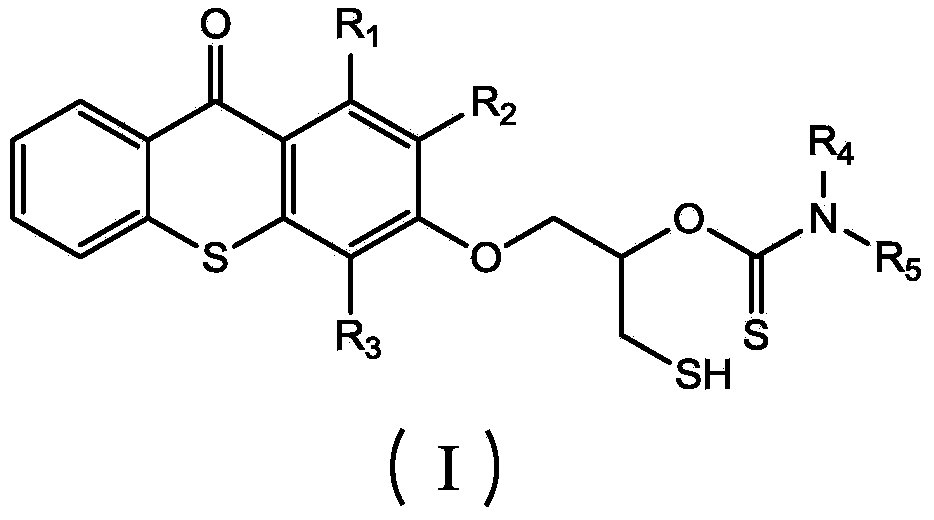
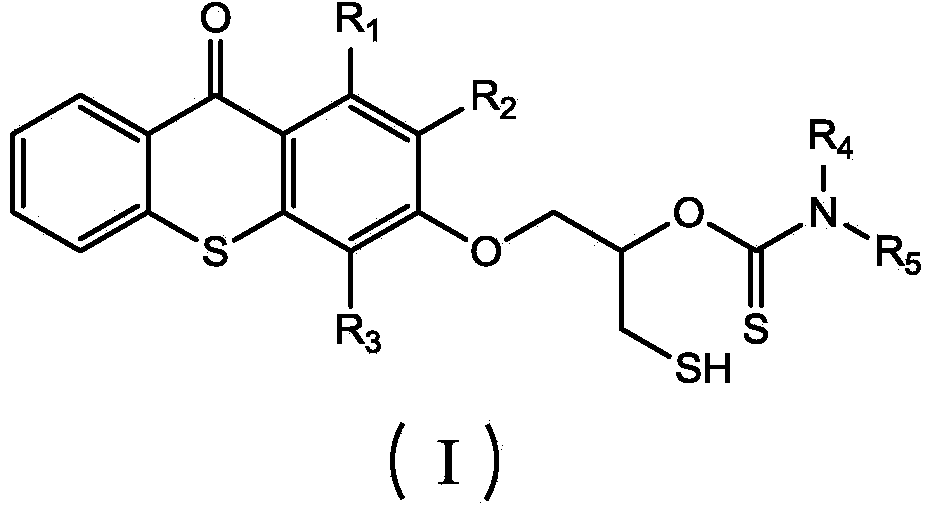
Thioxanthone photoinitiator containing sulfydryl and initiator aid amine and preparation method of thioxanthone
A technology of thioxanthone light and photoinitiator, which is applied in organic chemistry and other fields, and can solve problems such as hindering and unfavorable macromolecular photoinitiators
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
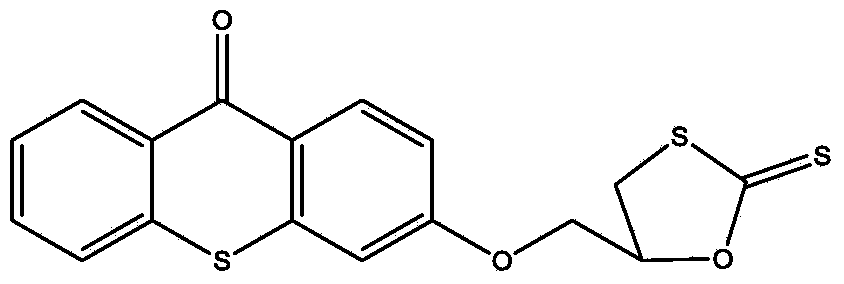
[0025] (1) Dissolve 1 mole of thioxanthone glycidyl ether in 0.2 L of THF solvent to form a solution, add 0.005 moles of catalyst lithium chloride and 1.2 moles of carbon disulfide to the solution, and react at -20°C for 1 hour , then react at 20°C, monitor the reaction process by infrared, when the characteristic peaks of epoxy groups at 823cm-1 and 910cm-1 disappear, the reaction is complete, pour the reaction solution into excess water, and extract with ethyl acetate , The solvent was removed under reduced pressure to obtain an intermediate product, which was detected by FTIR (KBr): 1191cm-1 (C=S), 1048cm-1 (C-O) and 643cm-1 (C-S);
[0026] (2) Add 1 mole of benzylamine dropwise to all the intermediate products obtained in step (1), and the dropwise addition is completed in 2 hours, and react at 30° C. for 3 hours. After the reaction is completed, thioxanthene containing mercapto and amine is obtained. Ketone photoinitiator, the product is detected: FTIR (KBr): 2600cm-1 (S-...
Embodiment 2
[0028] (1) Dissolve 1 mole of thioxanthone glycidyl ether in 0.6 L of NVP solvent to form a solution, add 0.05 moles of catalyst lithium bromide and 1.2 moles of carbon disulfide to the solution, react at 10°C for 4 hours, and then React at 40°C, monitor the reaction process by infrared, when the characteristic peaks of epoxy groups at 823cm-1 and 910cm-1 disappear, the reaction is complete, pour the reaction solution into excess water, extract with ethyl acetate, and decompress The solvent was removed to obtain an intermediate product, which was detected by FTIR (KBr: 1190 cm-1 (C=S), 1050 cm-1 (C-O) and 652 cm-1 (C-S).
[0029] (2) To all the intermediate products obtained in step (1), add 1 mole of methylbenzylamine dropwise, and the addition is completed in 2.5 hours, and react at 80° C. for 12 hours. Xanthone photoinitiator, the product is detected: FTIR (KBr): 2552cm-1 (S-H), 1168cm-1 (C=S), 1055cm-1 (C-O) and 3400cm-1 (N-H).
Embodiment 3
[0031] (1) 1 mole of thioxanthone glycidyl ether was dissolved in 0.3L of DMF solvent to form a solution, and 0.01 mole of catalyst lithium iodide and 1.2 mole of carbon disulfide were added to the solution, and reacted at 0° C. for 3.5 hours, Then react at 28°C, monitor the reaction process by infrared, when the characteristic peaks of the epoxy groups at 823cm-1 and 910cm-1 disappear, the reaction is complete, the reaction solution is poured into excess water, extracted with ethyl acetate, The solvent was removed under reduced pressure to obtain an intermediate product, which was detected by FTIR (KBr): 1195 cm-1 (C=S), 1051 cm-1 (C-O) and 644 cm-1 (C-S).
[0032] (2) To all the intermediate products obtained in step (1), add 1 mole of phenethylamine dropwise, dropwise in 3 hours, react at 60° C. for 5 hours, and complete the reaction to obtain thiol-containing and amine-containing thia Anthrone photoinitiator, the product is detected: FTIR (KBr): 2554cm-1 (S-H), 1163cm-1 (C...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com