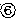Preparation method of folic acid modified Pluronic P85 copolymer and application of folic acid modified Pluronic P85 copolymer in 5-fluorouracil nano drug
A technology of fluorouracil and P85-NH2, which is applied in the field of pharmaceutical applications, can solve the problems of inconvenient clinical use, toxic and side effects, and restrictions on wide application, and achieve the effects of low toxic and side effects, growth inhibition, and good therapeutic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1. Synthesis of Folate-modified Pluronic P85 (P85-FA)
[0030]
[0031] (1) Synthesis of N,N'-carbonyldiimidazole (CDI)-activated Pluronic P85 (P85-CDI)
[0032]The synthesis of P85-CDI adopted the method in the literature ([1] W. Zhang, Y. Shi, Y. Chen, J. Ye, X. Sha, X. Fang, Multifunctional Pluronic P123 / F127 mixed polymeric micelles loaded with paclitaxel for the treatment of multidrug resistant tumors, Biomaterials, 32 (2011) 2894-2906.). Dissolve 1.0 g of Pluronic P85 (0.22 mmol) in 7 mL of dry acetonitrile, and slowly add dropwise to 7 mL of dry acetonitrile solution containing CDI (0.35 g, 2.15 mmol). Under the protection of nitrogen, the reaction was stirred overnight at room temperature. After the reaction was complete, the solution was concentrated under reduced pressure and poured into excess diethyl ether. Excess CDI was removed by centrifugation at a speed of 10,000 rpm, and the ether layer was collected. This step was repeated three times...
Embodiment 2
[0037] Example 2. Preparation of P85-FA / 5-Fu
[0038] Preparation of P85-FA / 5-Fu micelles: Using thin film hydration method, take 300 mg of P85-FA and a certain amount of 5-Fu in a 50 mL round-bottomed flask, dissolve them with an appropriate amount of acetonitrile, and heat them at 60 °C under 100 r / min rotary evaporation for 1 h to evaporate the organic solvent to dryness, and vacuum-dry for 5 h to remove the residual organic solvent to obtain a dry film. Heated in a water bath to dissolve the solid skeleton to obtain a transparent gel-like sample. Add 10 mL of isothermal purified water, stir at a constant speed for 30 min (700 r / min), and filter with a 0.22 μm membrane to obtain the P85-FA / 5-Fu micellar solution.
Embodiment 3
[0039] Example 3. Determination of P85-FA / 5-Fu Particle Size
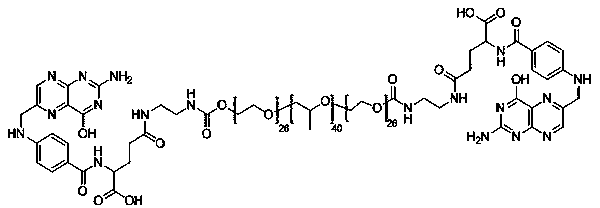
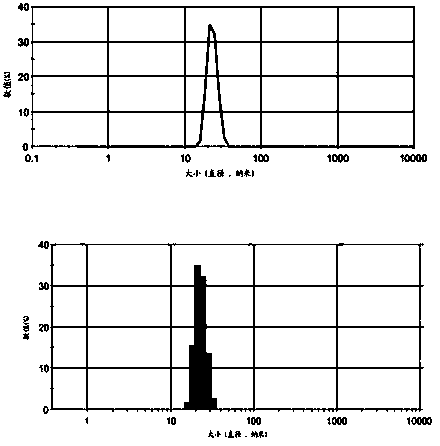
[0040] The particle size of P85-FA / 5-Fu was measured with a Malvern particle size analyzer. The particle size measurement parameters were as follows: He-Ne laser (wavelength 635 nm), refractive index and viscosity were n=1.330 and η=0.888 cp, and the measurement temperature at 25°C. The sample concentration was 0.4 mg / ml. The particle size of the constructed P85-FA / 5-Fu is between 10-100 nm. (see attached figure 2 )
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com