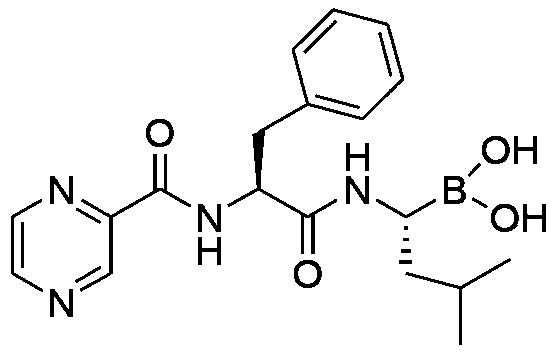
A kind of preparation method of bortezomib
A technology of bortezomib and borate ester, which is applied in the field of bortezomib synthesis, can solve the problems of increasing post-processing time and difficulty, increasing the risk of reaction, and complicated equipment, so as to save separation and purification time and reduce pollution of three wastes , the effect of simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0027] The invention provides a kind of preparation method of bortezomib, comprises the steps:
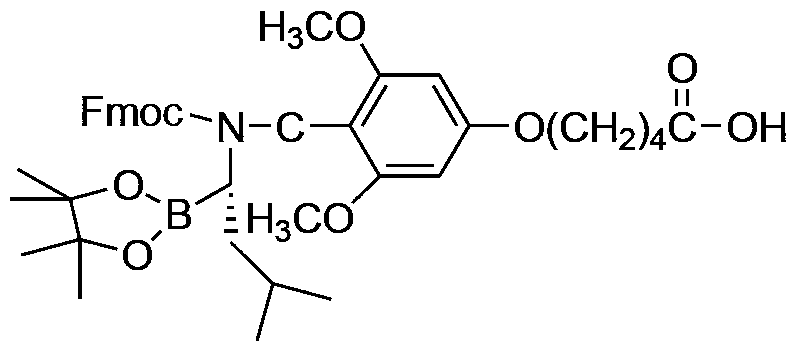
[0028] a. With pinacol-1-amino-3-methylbutane-1-boronate (compound II) and 5-(4-formyl-3,5-dimethoxyphenoxy)pentane The acid (compound I) was used as the starting material, and the intermediate product 3 was obtained through condensation, reduction, and Fmoc-protection reactions. The structural formula is as follows:
[0029]
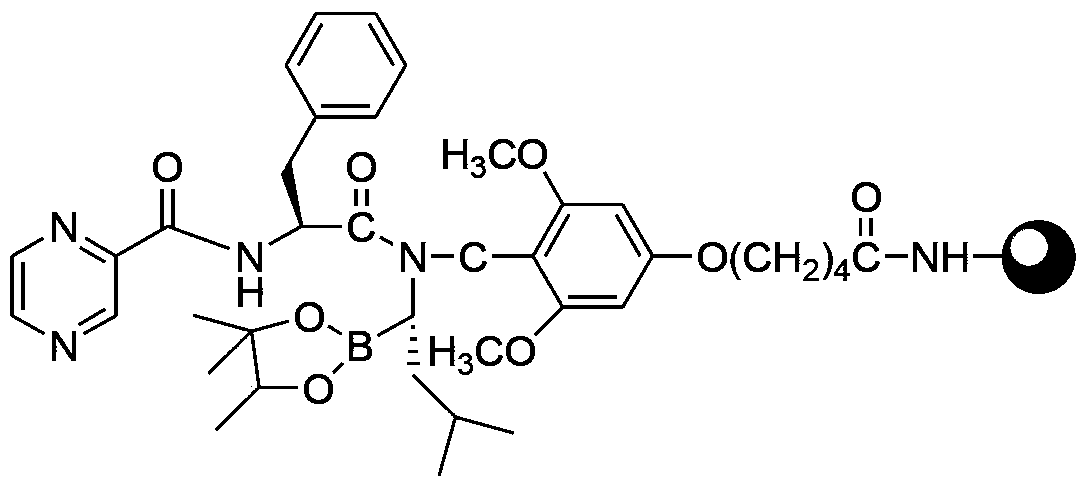
[0030] b. Using a resin as a solid phase carrier, sequentially couple the intermediate product 3 of step a, Fmoc-L-phenylalanine (compound III) and pyrazine-2-carboxylic acid (compound IV) to obtain resin compound 6 ;
[0031] c. Cutting the resin compound 6 of step b to obtain boronate 7 of bortezomib,
[0032] d. The product obtained in step c is hydrolyzed to obtain the final product.
[0033] step 1)
[0034] The intermediate product 3 can be prepared as follows:
[0035] a. Add pinacol-1-amino-3-methylbutane-1-boronate, 5-(4-formyl-3,5-dimethoxy...
Embodiment 1
[0069] The synthesis of embodiment 1 intermediate product 3
[0070] Weigh compound Ⅰ (14.2g, 50mmol), compound Ⅱ (10.6g, 50mmol) and NaBH3CN (0.3g, 50mmol) into a 500ml glass flask, add MeOH (500ml), dissolve and react at room temperature for 60 minutes. After the reaction, the above reaction solution was concentrated and vacuum-dried to obtain an oily compound. Afterwards the oily compound was dissolved with dioxane and water (1:1, 400ml) and solid NaHCO was added 3 (13g, 150mmol), stirring was continued for 30 minutes. Fmoc-Cl (16.2g, 60mmol) was dissolved in 100ml of dioxane, added to the above solution under ice-cooling conditions, kept stirring under ice-cooling for 90 minutes, and then continued stirring at room temperature for 90 minutes until the reaction was completed. The above reaction solution was solid NaHCO 3 Adjust to pH=9, add water (2000ml) to dilute, and use Et 2 O was extracted twice, the aqueous phase was collected, and the aqueous phase was acidified ...
Embodiment 2
[0071] The synthesis of embodiment two resin compound 4
[0072] Rink amide resin (40 g, substitution degree 0.5 mmol / g) was weighed and added to a solid-phase reaction column (purchased from Sichuan Shuniu), swelled with DMF for 30 minutes, and drained of DMF. Weigh HATU (15.5g, 40mmol), HOAT (6.0g, 40mmol) and the intermediate product 3 (28g, 40mmol) in Example 1 and dissolve it with DMF (120ml), add DIPEA (14ml, 80mmol) under ice bath conditions After activating for 5 minutes, it was added to the reaction column, detected by ninhydrin until the resin was colorless, the reaction was completed for 2 hours, and the resin was washed 5 times with DMF to obtain resin compound 4, which was directly carried out in the next step.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com