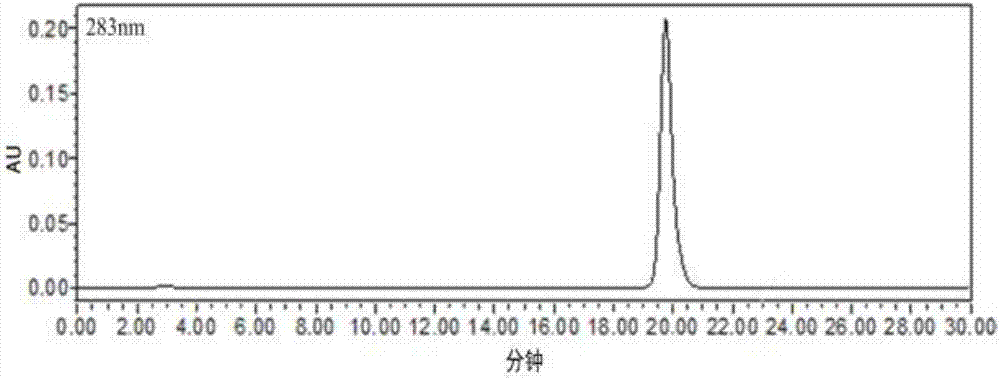
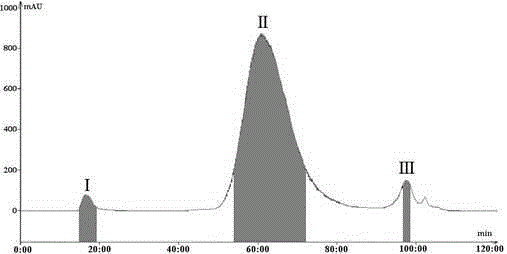
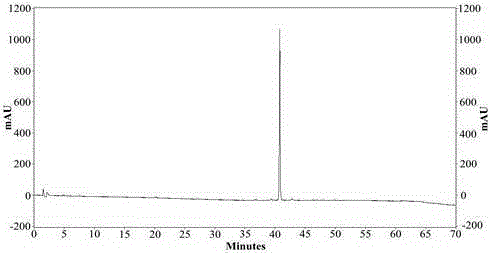
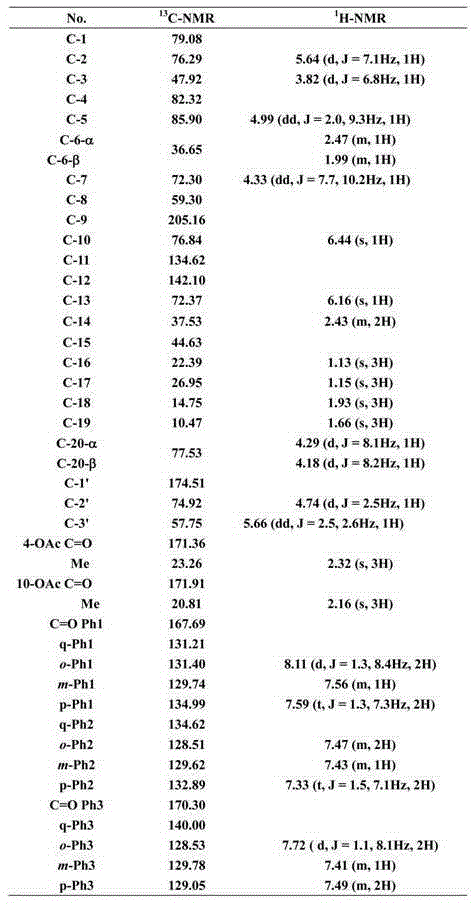
Patents
Literature
30results about How to "Shorten separation and purification time" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Method for separating and purifying oligodendrocyte precursor cells
InactiveCN101735983AAvoid damageIncrease acquisition rateNervous system cellsSingle cell suspensionWhite matter
The invention discloses a method for separating and purifying oligodendrocyte precursor cells. The method comprises: digesting cerebral cortex of neonatal rats and part of white matter with trypsin and DNA enzyme; blowing and beating the obtained product to be a single-cell suspension; inoculating the single-cell suspension into a culture flask coated with polylysine in advance by use of a mixed-cell culture medium; performing culture for 3 to 5 days; replacing the culture flask with an oligodendrocyte precursor cell proliferation culture medium when the fusion of bottom-layer cells reaches 65 to 75 percent; performing culture for 3 to 5 days; replacing the oligodendrocyte precursor cell proliferation culture medium with an oligodendrocyte precursor cell separation culture medium; digesting the obtained product at 37 DEG C; isolating oligodendrocyte precursor cells from mixed cells; blowing and beating the obtained product to be the single-cell suspension; inoculating the single-cell suspension into the culture flask coated with polylysine in advance by use of an oligodendrocyte precursor cell inoculation culture medium; replacing the culture flask with an oligodendrocyte precursor cell purification culture medium after the cells adhere to a wall; performing culture for 2 to 4 days; and obtaining adherence cells, namely the oligodendrocyte precursor cells. The method can obtain a large number of high-purity good-activity oligodendrocyte precursor cells conveniently, rapidly, economically and efficiently.
Owner:ARMY MEDICAL UNIV
Industria production method for separating and purifying chicken eimeria coccidium oocyst
InactiveCN103881913AShorten separation and purification timeShorten the timeProtozoaMicroorganism based processesBiotechnologyVaccine Production
The invention discloses an industrialized production method for separating and purifying a chicken eimeria coccidium oocyst. According to the industrialized production method, the separation and purification of the chicken eimeria coccidium oocyst are carried out by adopting the scientific assembly of a disc separator and a tubular type centrifuge, after sporulated oocysts are purified, larger impurities and extremely tiny impurities are not generated; an object plate is thoroughly subjected to microscopic examination, unsporulated oocysts are also all thoroughly removed, sporulated oocysts are all in the visual field, and the solution background is clear. The industrial production method disclosed by the invention can be used for shortening the separation and purification time of the oocysts; compared with the traditional technology, the industrial production method disclosed by the invention can be used for saving the time by more than 70%, reducing the product instability caused by excessive human factors by preventing integral oocyst purification from the disturbance of excessive artificial working and is the breakthrough of the existing coccidium vaccine production method; the industrial production method can be used for continuous production by only needing two centrifuges, thereby greatly reducing the cost input of an enterprise.
Owner:INST OF ANIMAL HEALTH GUANGDONG ACADEMY OF AGRI SCI
Method for separating and purifying paclitaxel from taxus chinensis branches and leaves or bark
The invention discloses a method for separating and purifying paclitaxel from taxus chinensis branches and leaves or bark. The method comprises the following steps: firstly, immersing taxus chinensis branches and leaves or bark by methanol, carrying out ultrasonic extraction or reflux extraction, and concentrating at reduced pressure to obtain an extract; secondly, and extracting the extract sequentially by petroleum ether and ethyl acetate to obtain an ethyl acetate extract; thirdly, carrying out chromatography on the ethyl acetate extract through an alkaline aluminum oxide column to obtain a paclitaxel-enriched component, and treating the paclitaxel-enriched component by utilizing a partial precipitation method which uses methanol-water as a system to obtain a crude paclitaxel product; and finally, refining to obtain paclitaxel with purity over 98.5 percent by using flash chromatography as a separation means and methanol-water as an eluting system. The method is simple and convenient to operate, has the advantages of low cost, good repeatability, high efficiency and high product purity, and can be used for large-scale production of paclitaxel.
Owner:SUN YAT SEN UNIV
Digestive juice and method for dispersing pancreatic cancer tissue of human into single living cells
InactiveCN109554345ALow costEasy to operateCell dissociation methodsTumor/cancer cellsDispaseInstrumentation
The invention relates to digestive juice and method for dispersing pancreatic cancer tissue of human into single living cells, and belongs to the field of unicellular analysis and cell culture. The digestive juice prepared by mixing three enzymes of Collagenase Type IV, Dispase and DNase I is mainly used for treating pancreatic cancer tissue, and then operations of performing centrifugation, removing dead cells and the like are performed, so that single pancreatic cancer cells high in purity and high in activity can be obtained within a short time. The used reagent is a frequently-used molecule cell biology reagent. Used consumptive materials are also common cell culture consumptive materials, are low in price and are easy to obtain, so that the cost is effectively reduced. The entire operation process is simple, special instruments and equipment are not needed, the operation process can also be performed in a general purpose laboratory, and the method has high practicality.
Owner:SINGLERON NANJING BIOTECHNOLOGIES LTD
Rapid separation and purification method of cordyceps militaris fruit body water-soluble peptide polysaccharide
InactiveCN101200491AAvoid collectingSimple processPeptide preparation methodsOn columnPurification methods
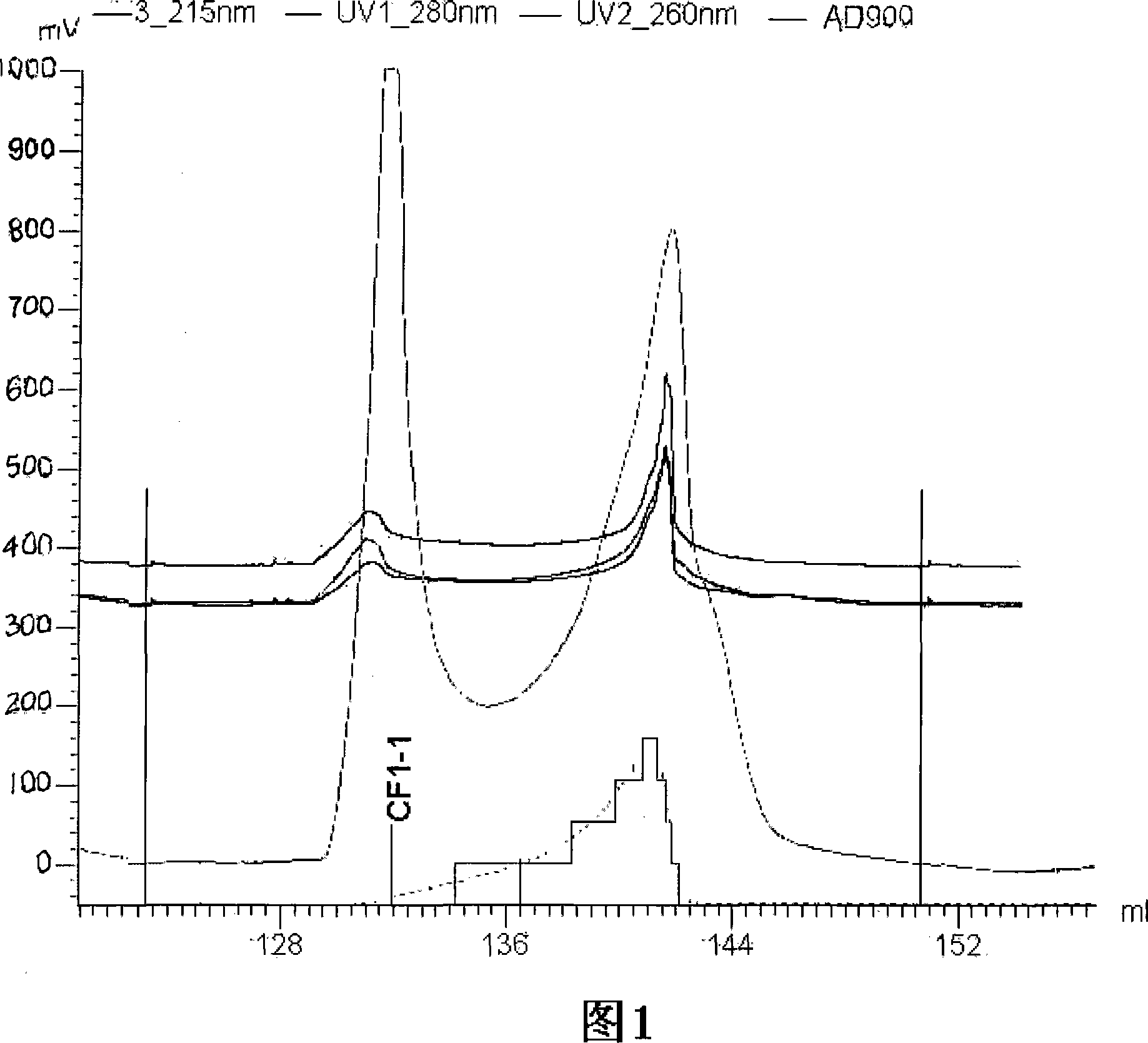
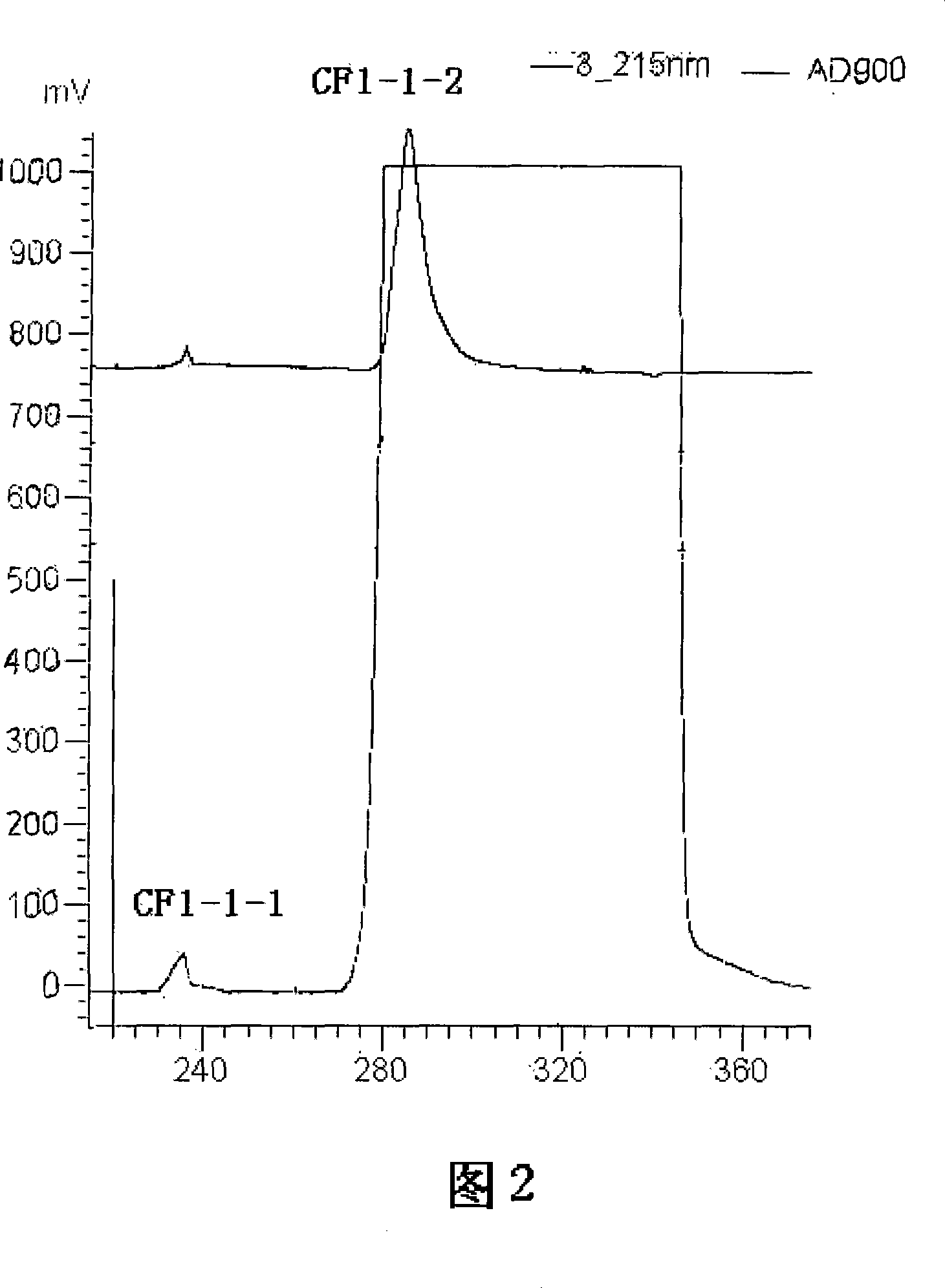
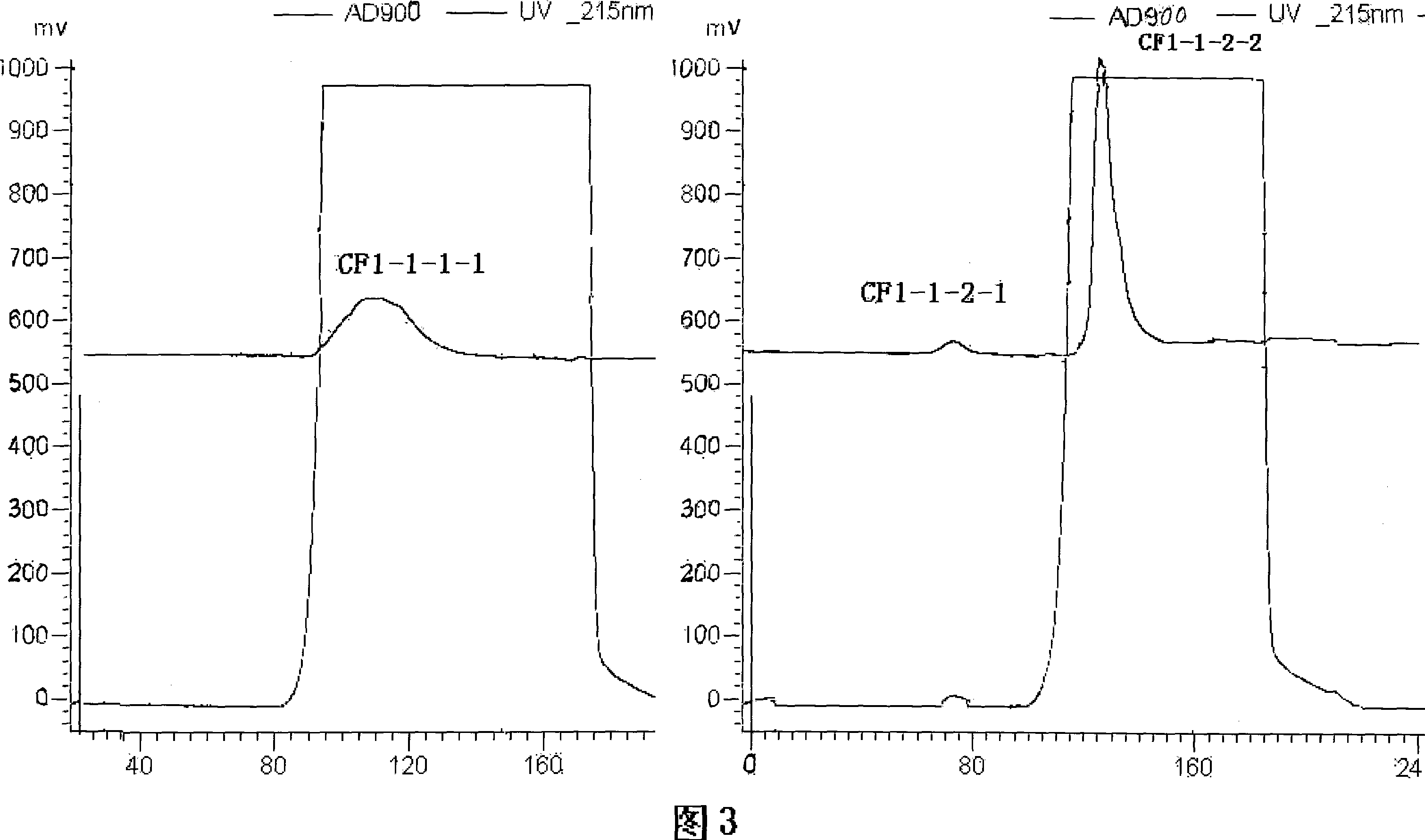
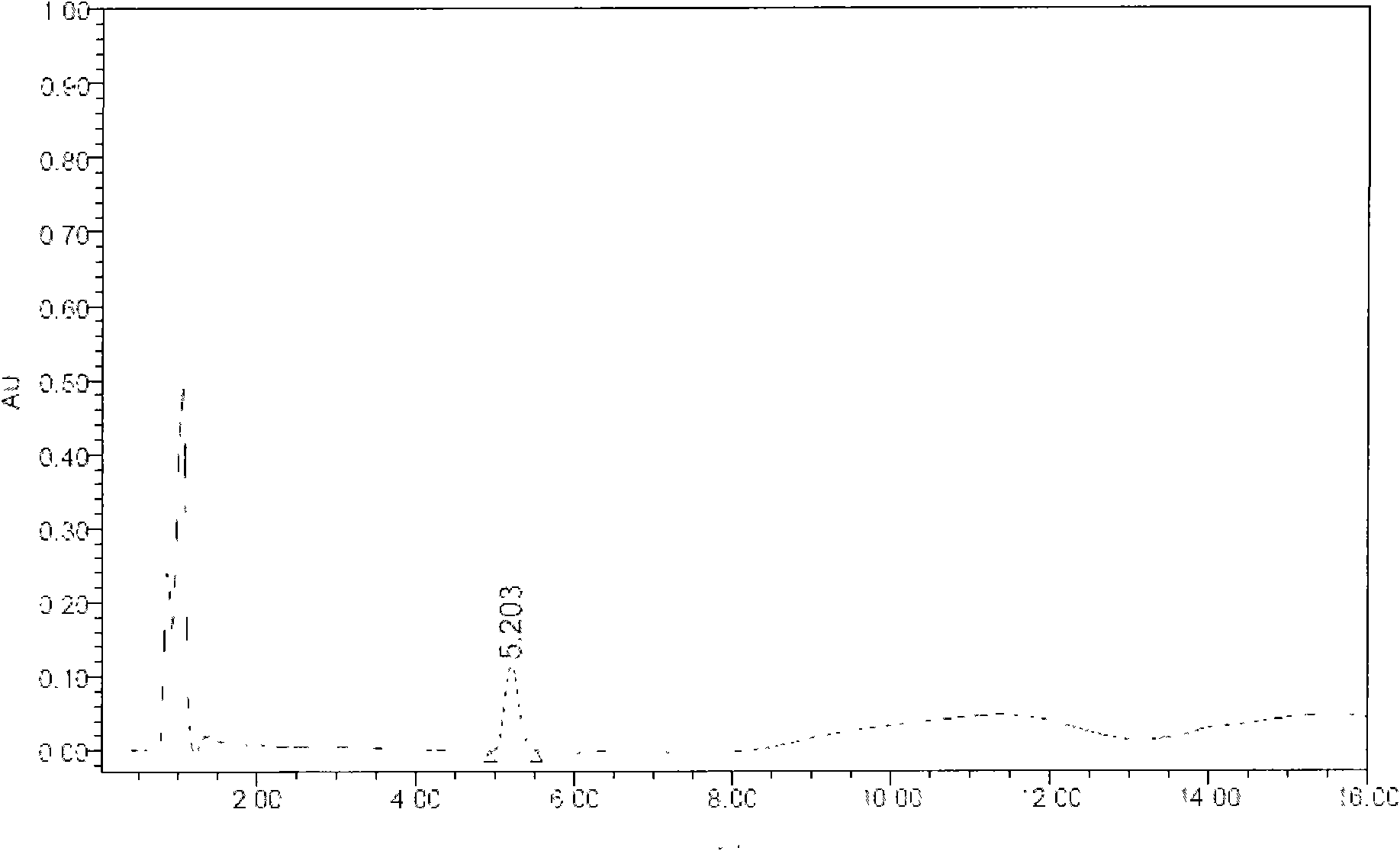
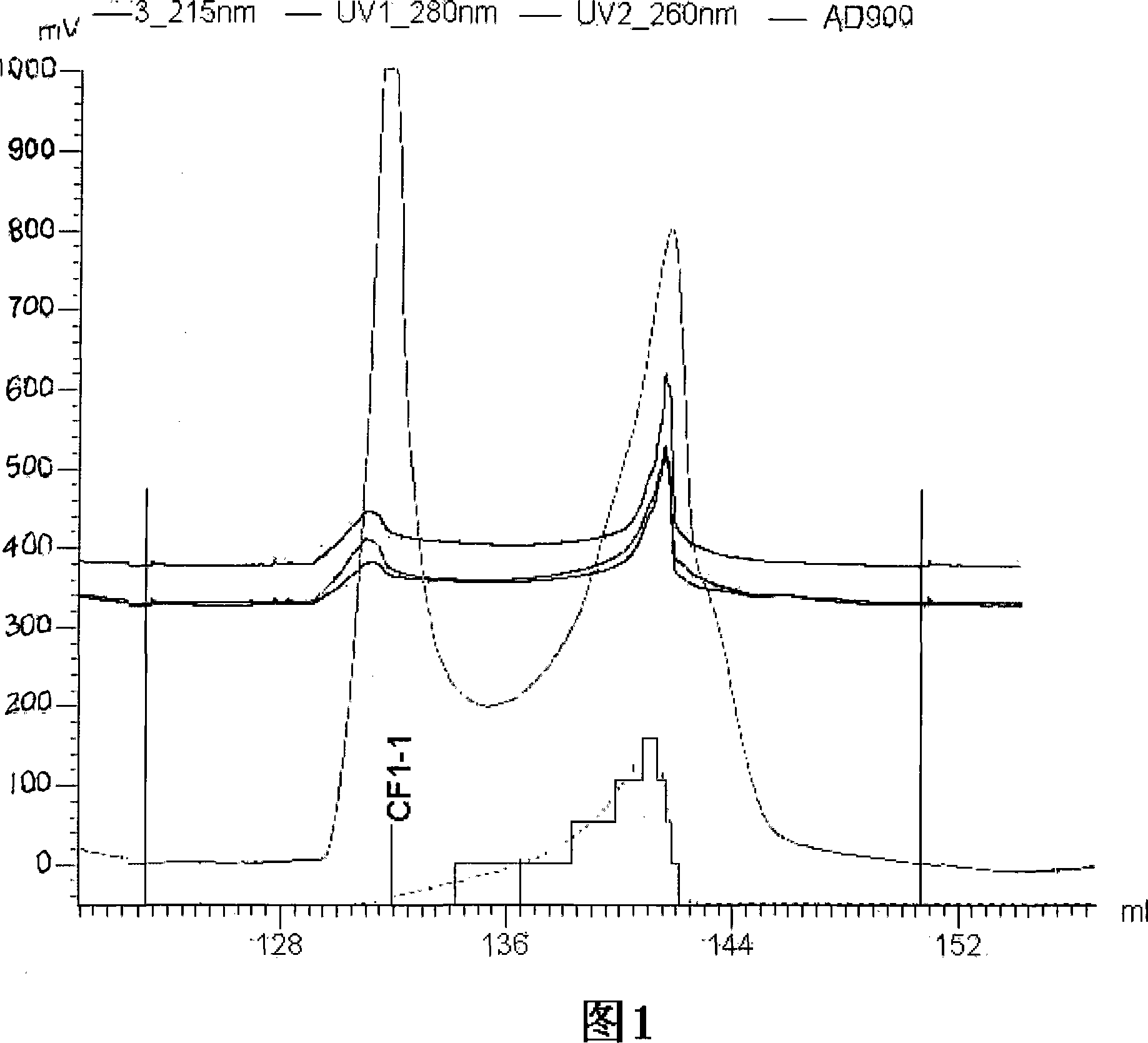
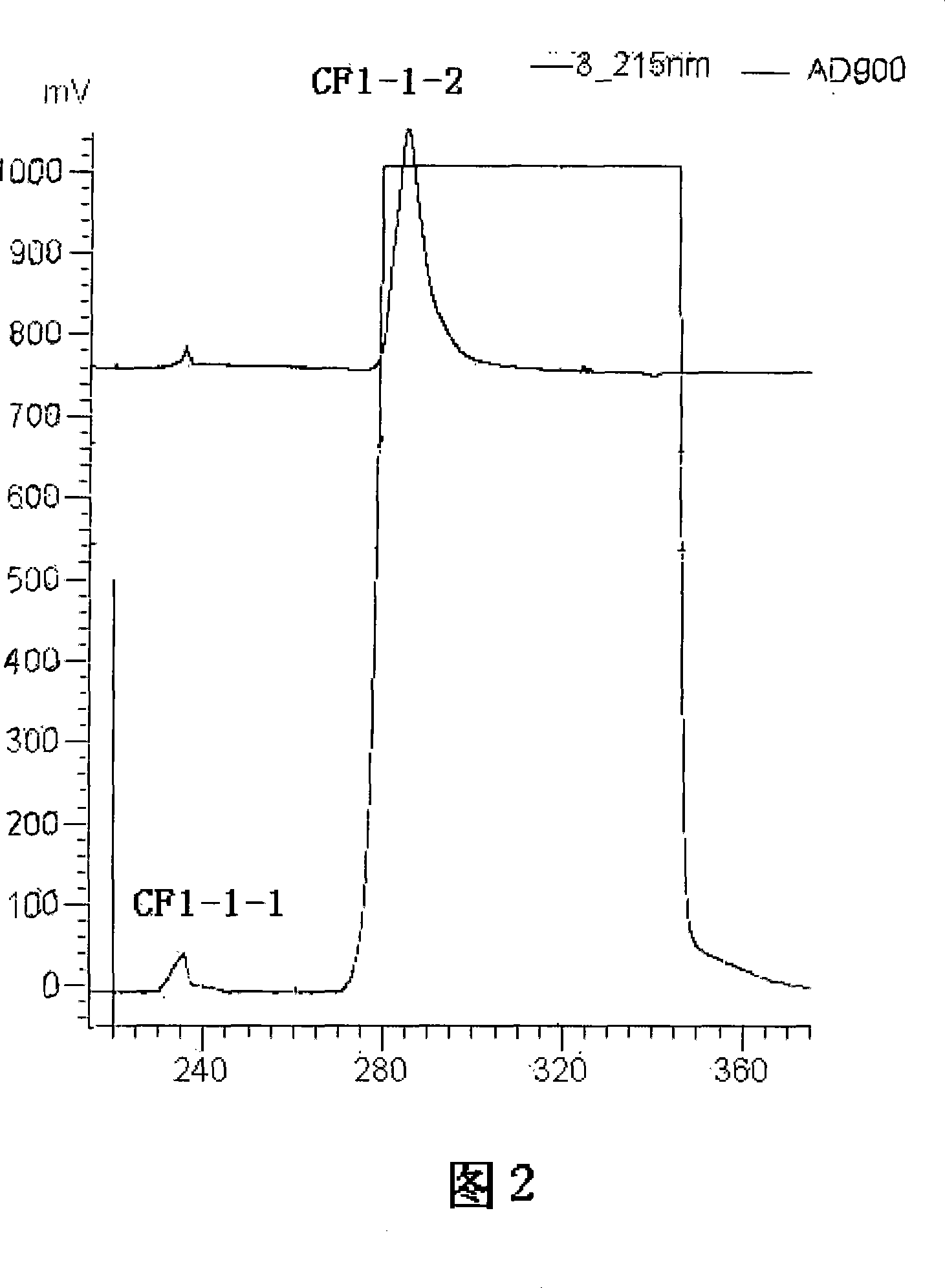
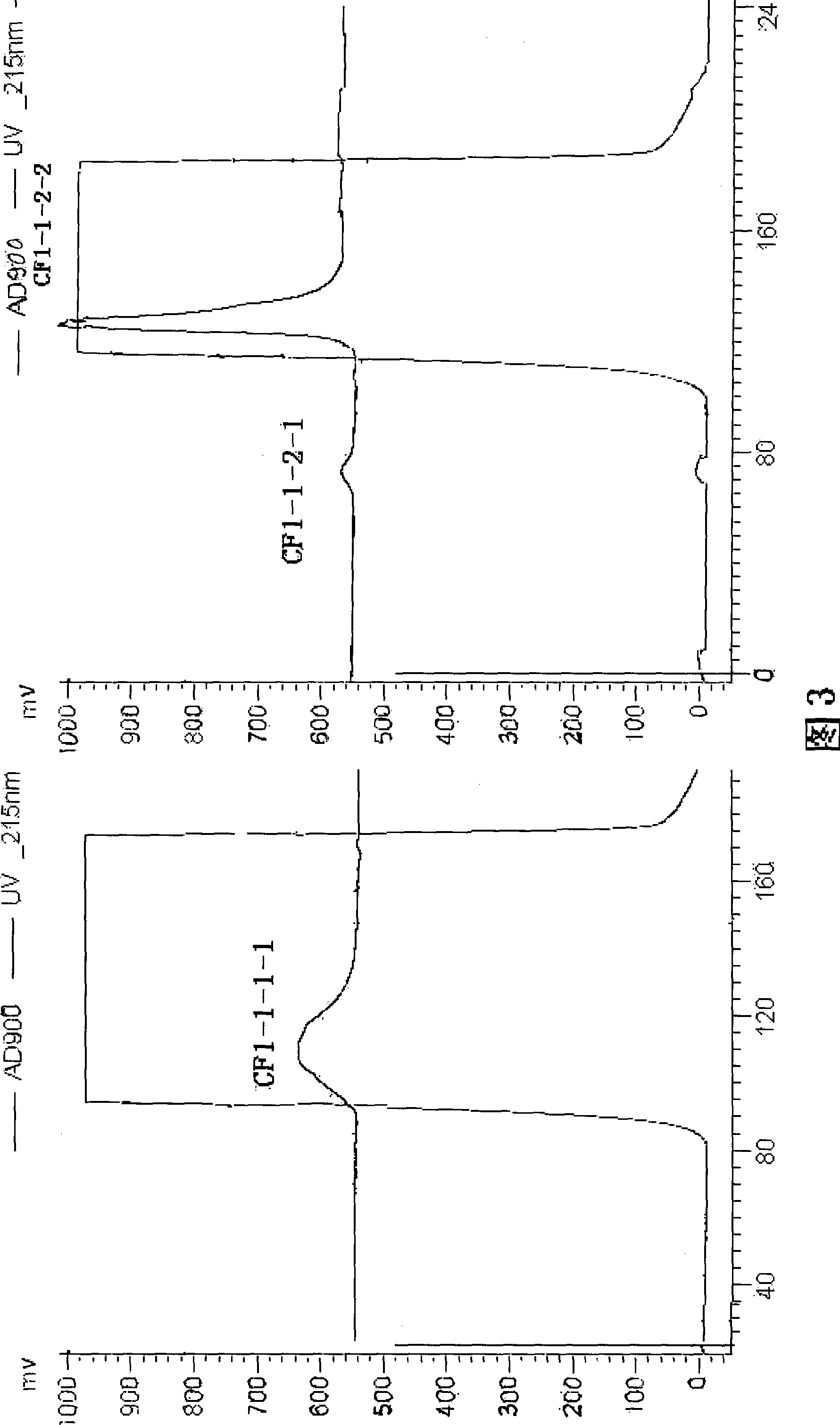
The invention discloses a rapid separation and purification method of the water soluble peptide polysaccharide from Cordyceps Militaris and belongs to the extraction technology field of the bioactive substances. The method comprises drying and smashing the Cordyceps Militaris, obtaining the component CF1 by extracting the ultrapure water, obtaining the component CF1-1 by the separation from the molecular sieve, obtaining the component CF1-1-1 and CF1-1-2 by separation and purification from the ionic exchange column, and obtaining the CF1-1-1-1, CF1-1-2-1 and CF1-1-2-2 by separation from the affinity chromatography column. By adopting the purification method of smashing the Cordyceps Militaris and direct introduction of the water extract on column, the invention greatly promotes the efficiency and decreases the cost, in addition, the entire separation and purification process does not need the organic solvent, thereby really achieving the purpose of green, rapidness and high efficiency of the separation of the polysaccharide. The method can be widely applied in the separation and purification technology of the water soluble peptide polysaccharide from the Cordyceps Militaris in the factory and the laboratory.
Owner:ZHEJIANG ACADEMY OF AGRICULTURE SCIENCES
Method for removing proteins and pigments in ganoderma lucidum crude polysaccharide
ActiveCN101724088AShorten separation and purification timeSolving step-by-step removal of protein from crude polysaccharidesOrganic solventElution
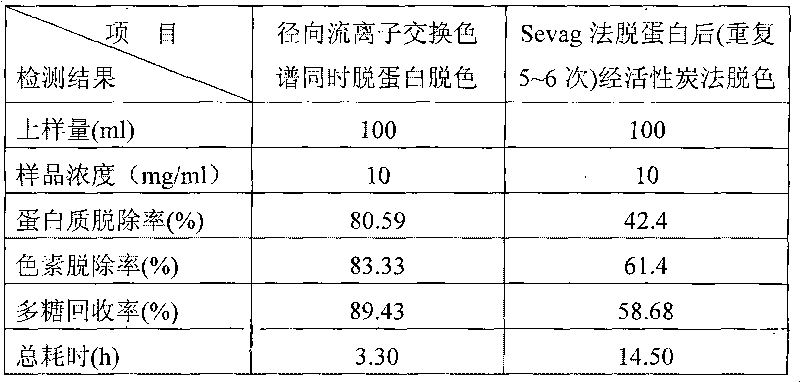
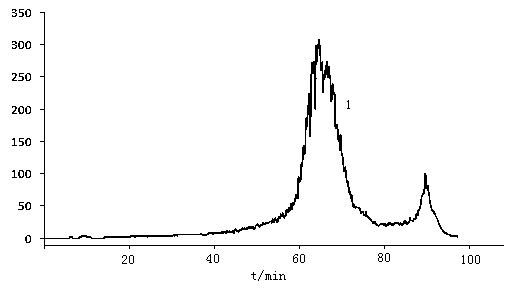
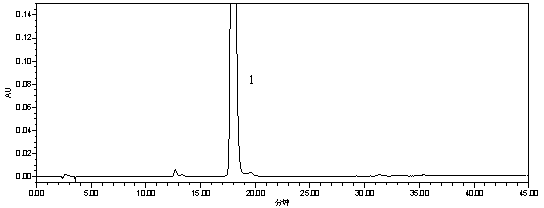
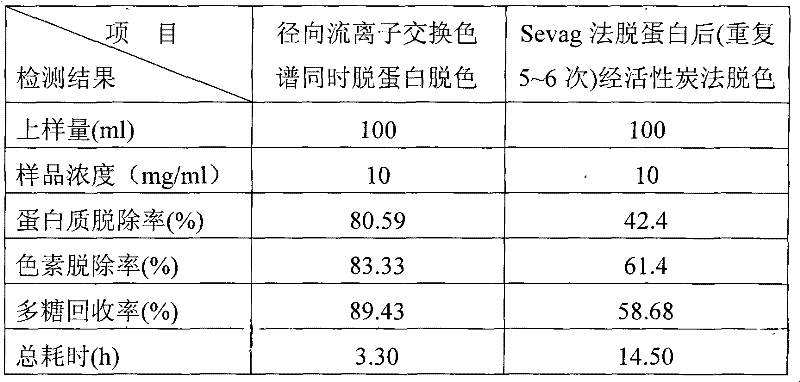
The invention discloses a method for removing proteins and pigments in ganoderma lucidum crude polysaccharide, which comprises the following steps of: preparing the ganoderma lucidum crude polysaccharide into 10-30mg / m1 aqueous solution; centrifuging the aqueous solution to take a liquid supernatant; sampling the liquid supernatant; adding the liquid supernatant into a radial flow chromatographic column filled with an anion exchanger; then eluting the chromatographic column with pure water to control a sampling flow velocity to 2-10ml / min and an elution flow velocity to 10-50ml / min; collecting an eluent until no saccharide is detected in the eluent; combining the collected eluent; and carrying out post-treatment to obtain a pure product of the ganoderma lucidum polysaccharide. The method has the advantages that: the proteins and the pigments in the ganoderma lucidum crude polysaccharide are removed simultaneously so as to greatly shorten separation and purification time of the polysaccharide, be favorable for reserving the polysaccharide activity maximally and improve the purification efficiency of the ganoderma lucidum crude polysaccharide; organic solvents are not used in an operation process, conditions are mild, subsequent treatment is convenient, and environmental pollution is lightened; and the iron exchange resins have low cost and can be recycled so as to effectively reduce the production cost.
Owner:ZHEJIANG UNIV OF TECH
Preparation method of magnetic covalent organic framework molecularly imprinted polymer for separating anthocyanin
InactiveCN111269454AGood effectMagnetically responsiveOther chemical processesAlkali metal oxides/hydroxidesPolymer scienceMolecularly imprinted polymer
The invention discloses a preparation method of a magnetic covalent organic framework molecularly imprinted polymer for separating anthocyanin. The invention belongs to the field of molecularly imprinted polymers. The invention aims to solve the technical problem of low selectivity of the traditional separation filler for anthocyanin separation in the prior art. The method disclosed by the invention comprises the following steps: 1, synthesizing aminated magnetic nano Fe3O4-NH2; and 2, synthesizing seven magnetic covalent organic framework molecularly imprinted materials (MCMIPs) with C3G as atemplate molecule through a room-temperature synthesis method. The MCMIPs provided by the invention have magnetic responsiveness, high adsorption performance and excellent specificity, and are suitable for rapidly and purposefully fishing C3G from a complex sample matrix. According to the separation medium, the anthocyanin separation and purification steps are simplified, the separation and purification time is shortened, and the separation and purification efficiency is greatly improved.
Owner:HARBIN INST OF TECH
Radial flow chromatographic separation and purification method for biological polysaccharide
InactiveCN101914165AHigh purityLow pigment contentIon-exchange process apparatusIon-exchanger regenerationChromatographic separationPurification methods
The invention relates to a radial flow chromatographic separation and purification method for biological polysaccharide, which comprises the following steps of: centrifuging a medical fungi liquid fermentation product or a mycelium (fruiting body) enzymolysis product and collecting supernatant or collecting supernatant which comprises polysaccharide and is extracted from plants, animals, bacteriaand yeast; directly loading the supernatant on a macroporous resin radial chromatographic column of 500ml at the flow rate of 60 to 120ml per minute; eluting the macroporous resin radial chromatographic column by using distilled water or deionized water at the flow rate of 50 to 200ml per minute; and collecting 500 to 800ml of eluent, namely solution containing purified polysaccharide. The polysaccharide is directly separated and purified from the enzymolysis supernatant and the fermentation supernatant of the medical fungi mycelium and the supernatant extracted from the plants, animals, bacteria and yeast by a one-step method; and the biological polysaccharide separation and purification method has the advantages of simple process, convenient operation, no use of an organic solvent, highproduction efficiency and obvious purification effect.
Owner:HARBIN INST OF TECH AT WEIHAI
Process for extracting artemisinin from artemisia apiacea
The invention relates to a process for extracting artemisinin from artemisia apiacea. The process uses 200# gasoline as an organic solvent, and extracts by a reflux extracting method and a pressurizing cool circulating extracting method, wherein the process comprises steps like crushing, organic solvent extraction, recycling solvent, hot melting, decoloration, concentration, separating crystals and refining. In comparison with the traditional process, the process provided by the invention has advantages of lower production cost, a simpler process, a higher yield and is more suitable for modern mass production. The process shortens the separation purification time, and raises the purity, quality and stability of the product by cooperating an ion exchange resin and a macroporous absorption resin.
Owner:曾科
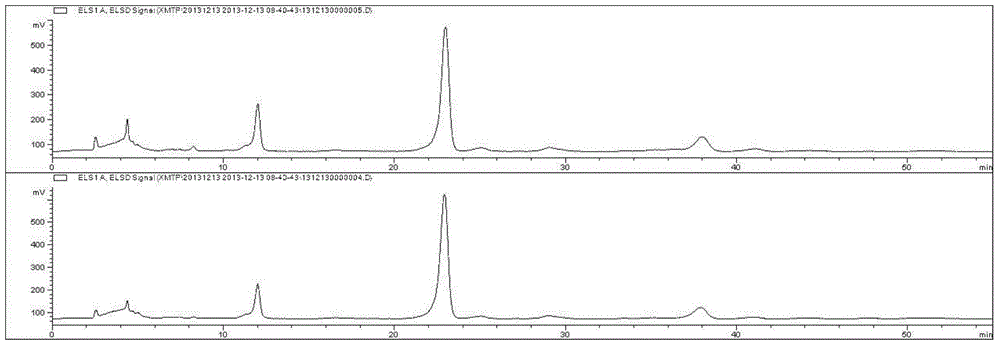
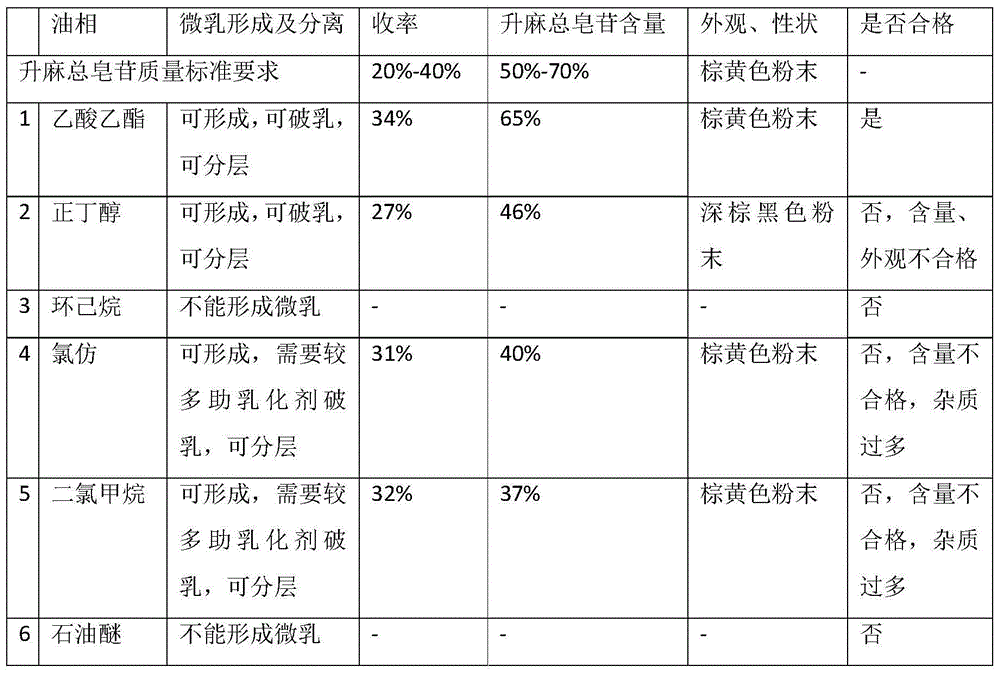
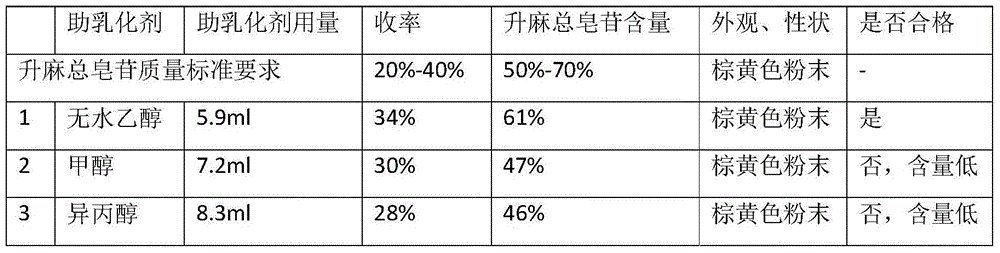
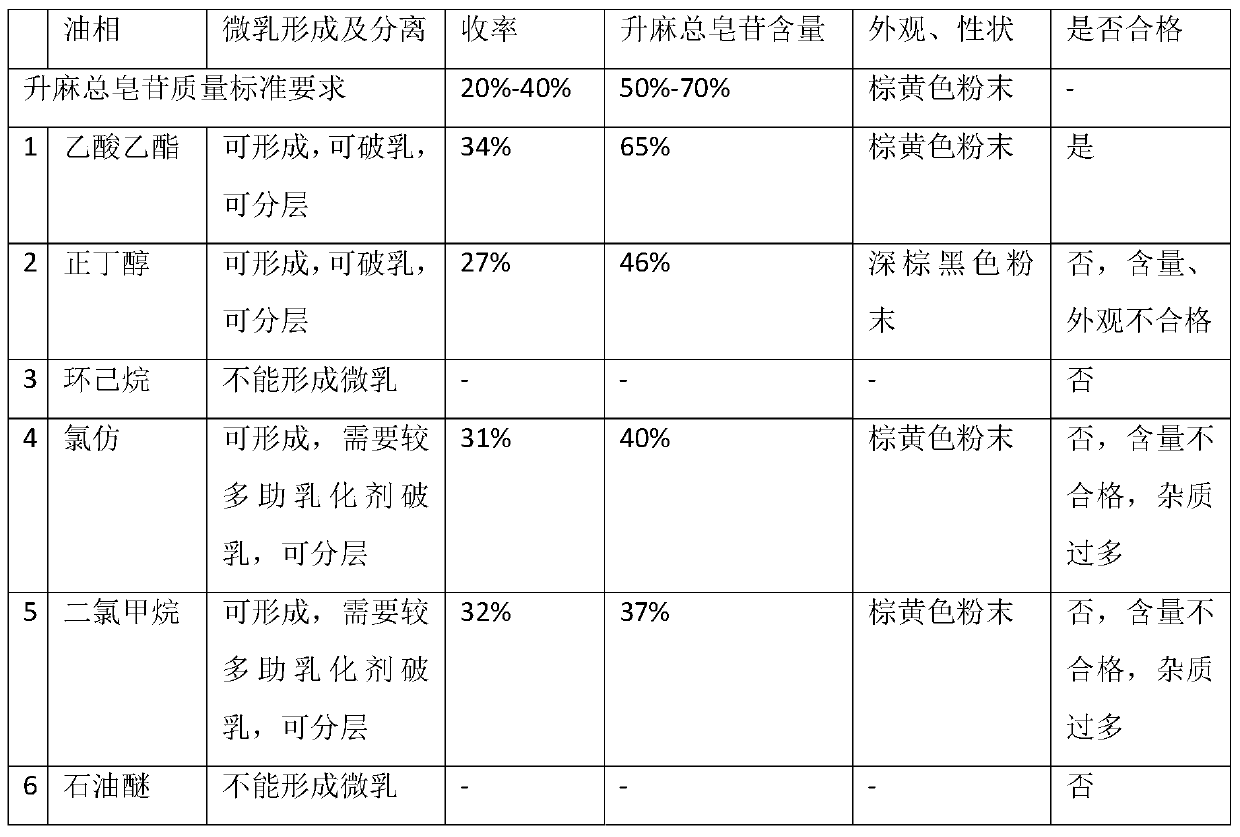
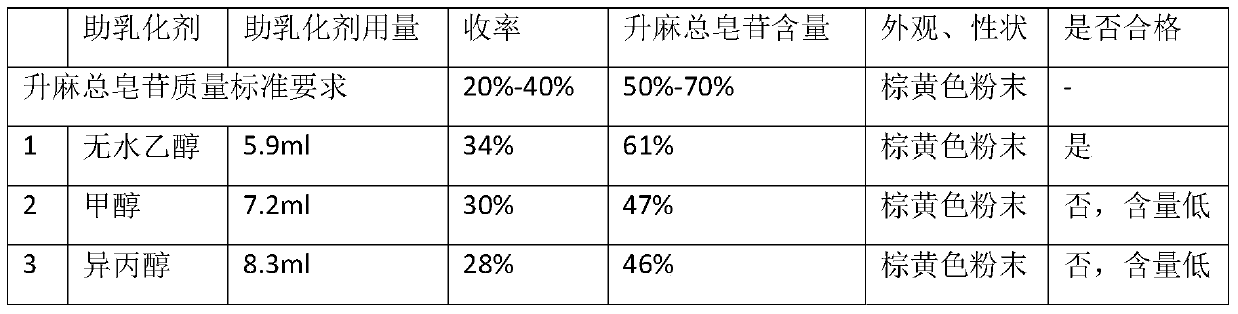
Preparation method of total saponins of rhizoma cimicifugae
The invention provides a method for preparing total saponins of rhizoma cimicifugae through separation and purification by virtue of a micro emulsion-phase interface extraction technique. The method can achieve the purpose of realizing industrial mass production by virtue of the micro emulsion extraction technique, and the method can save time and reduce the use of an organic solvent; and in addition, the clinical efficacy of the total saponins of rhizoma cimicifugae prepared by the method is better than that of total saponins of rhizoma cimicifugae obtained by a conventional separation and purification method.
Owner:SHANDONG LUYE PHARMA CO LTD
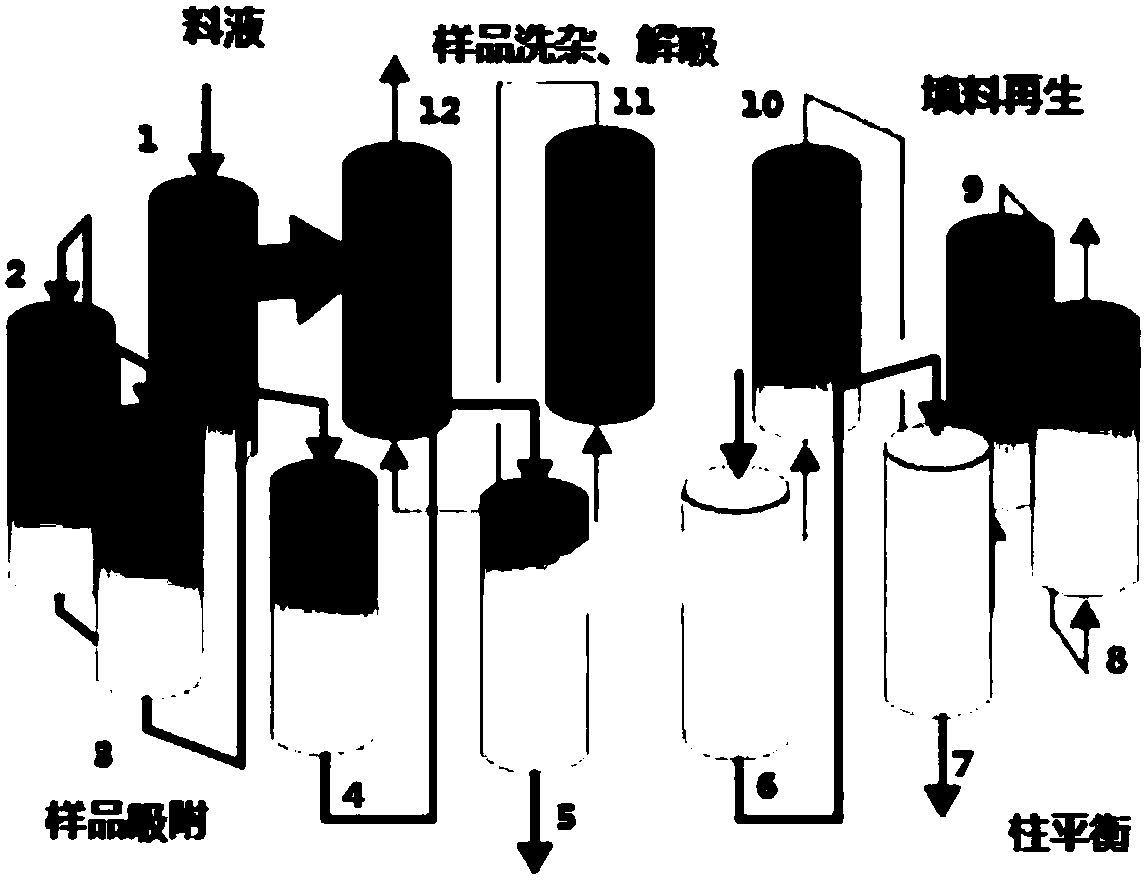
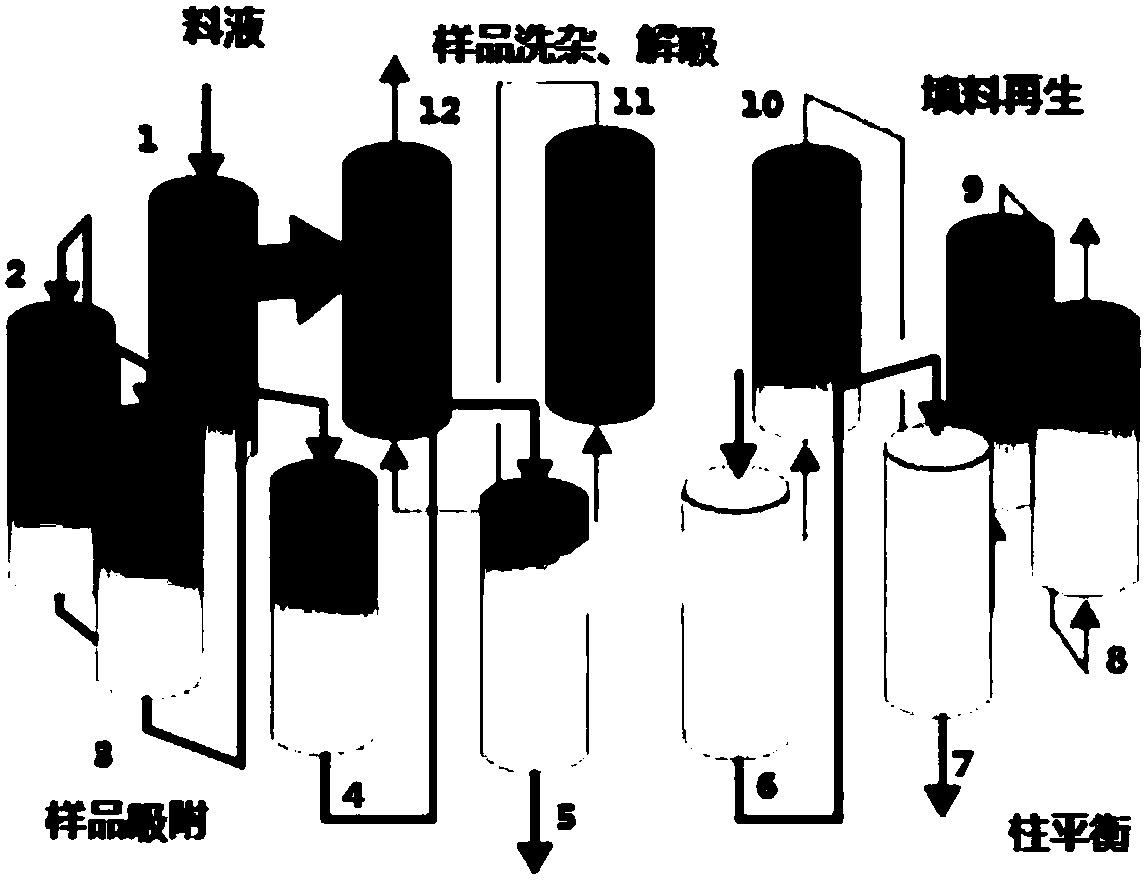
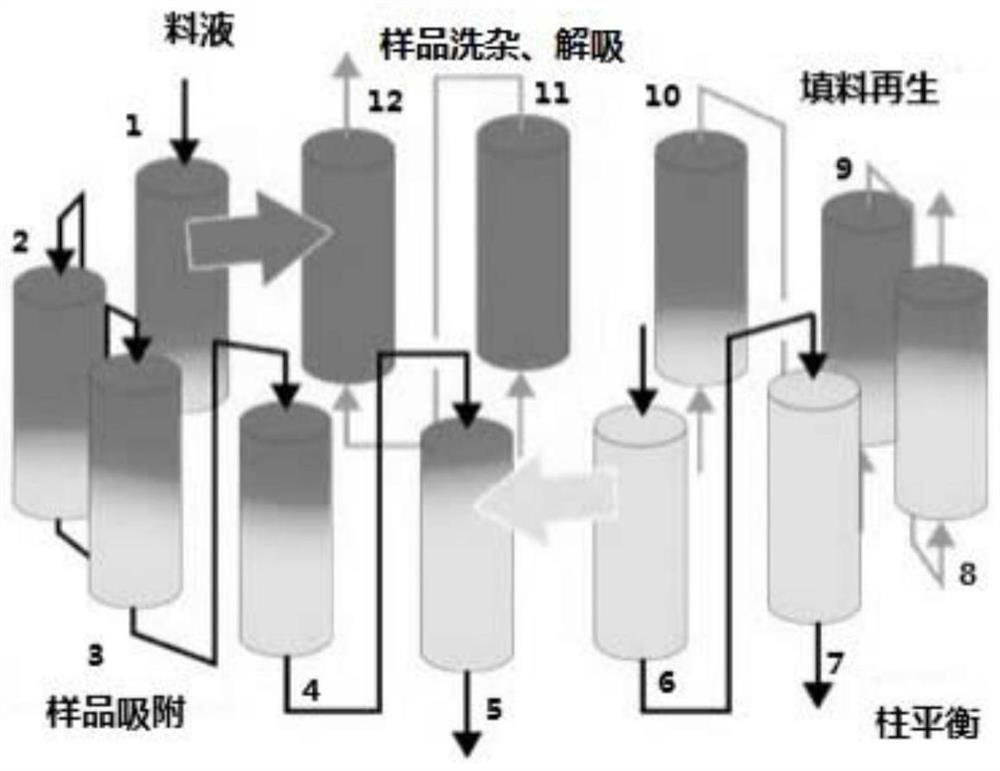
Method for separating and purifying FMD inactivated virus antigen by applying simulated flow bed
ActiveCN109836479AImprove continuityEasy to operateVirus peptidesPeptide preparation methodsDesorptionElution
The invention relates to a method for separating and purifying an FMD inactivated virus antigen by applying a simulated flow bed, wherein the method comprises the following steps: balancing a chromatographic column of the simulated flow bed with a balanced buffer solution; loading an FMD inactivated virus antigen sample into the chromatographic column of the simulated flow bed, during sample loading, simultaneously carrying out antigen sample impurity cleaning and sample desorption elution as well as chromatographic filler cleaning and regeneration and balanced liquid balancing on the chromatographic column, and continuously desorbing and eluting the collected and purified sample in the process. The simulated flow bed is applied to separation and purification of the FMD inactivated virus antigen for the first time, the continuous saturated sample loading separation operation can be realized and the sample loading amount is greatly improved; moreover, through the continuous separation and purification via multiple monomer columns, the degree of continuity is high, the separation and purification time is greatly shortened, the preparation efficiency is improved and the operation costis reduced. Moreover, the rigid filler is selected specifically, the loading capacity of the filler namely the pressure resistance is greatly improved, the use amount of the chromatographic filler isreduced, and the service life of the chromatographic column in the simulated flow bed is prolonged.
Owner:SHANGHAI SHEN LIAN BIOMEDICAL CORP
Method for separating reference substance of ergosterol and stellasterol from traditional Chinese medicine polyporus umbellatus
ActiveCN107216365ASmall difference in propertiesDifficult to separateOrganic chemistry methodsSteroidsMedicinal herbsMaterial resources
The invention discloses a method for separating a reference substance of ergosterol and stellasterol from a traditional Chinese medicine polyporus umbellatus. According to the method, high-purity monomeric compounds of the ergosterol and the stellasterol are prepared from polyporaceae polyporus polyporus(pers.) dry sclerotoids, serving as raw materials, by means of ethanol liquid extraction, petroleum ether decontamination, dichloromethane extraction, sephadex LH-20 column chromatography, re-crystallization and half-preparation. The method is simple and reliable, the raw materials are easy to obtain, the economic cost is low, the specificity is strong, and the method is applicable to large-scale preparation of the reference substance of the ergosterol and the stellasterol; moreover, during extraction and crystallization, ethyl alcohol or methyl alcohol is adopted to reduce the environmental pollution, and the solvent cost is low; by means of the crystallization or preparation method, the separation and purification time of a sample is shortened, the costs of manpower and material resources are reduced, and the economic effect is improved; a reference substance for content determination can be provided for quality control over crude drugs of the traditional Chinese medicine polyporus umbellatus, thus contributing to establishment of a quality control method for the crude drugs polyporus umbellatus.
Owner:GUANGDONG HOSPITAL OF TRADITIONAL CHINESE MEDICINE
Separation and purification method for maize pollen
ActiveCN104970279AShorten separation and purification timeHigh extraction rateCosmetic preparationsFood freezingPurification methodsFiltration
The invention provides a process method for separating and purifying maize pollen from maize anther. Maize male spikes (the male spikes are extracted and are close to dusting period) are extracted cross the ridge, and anther on the male spikes is rubbed down. The maize anther comprises two parts: pollen capsule walls and pollen wrapped thereby. The pollen and the pollen capsule walls are initially separated by water-soaking and filtering methods, the tissues of the filter residues (the pollen capsule wall) are titrated, and the titrated pollen capsule walls are further soaked in water and filtered so as to further separate the pollen from the pollen capsule walls. The filtrates of filtration twice are mixed together to obtain relatively pure pollen, and the filtrate is filtered, so that the effect of eliminating fine impurities and purifying the maize pollen can be achieved, and the filtrate is lyophilized to obtain pure maize pollen. The method is simple, feasible and high in efficiency, the nutrients of the maize pollen are not destroyed and the maize pollen is not damaged, and the method is suitable for scaled production.
Owner:SICHUAN UNIV
A method for separating and purifying paclitaxel from yew branches and leaves or bark
The invention discloses a method for separating and purifying paclitaxel from yew branches and leaves or bark. The method firstly soaks the branches, leaves or bark of the yew with methanol, ultrasonically extracts or reflux extracts, and concentrates under reduced pressure to obtain the extract; the extract is sequentially extracted with petroleum ether and ethyl acetate to obtain the ethyl acetate extract; Ethyl acetate extract was subjected to basic alumina column chromatography to obtain paclitaxel-rich components, and then treated with methanol-water as a system to obtain crude paclitaxel, and finally used rapid preparative chromatography as a separation method to obtain Methanol-water was used as the elution system, and paclitaxel was finally refined (purity>98.5%). The method of the invention has the advantages of simple operation, low cost, good repeatability, high efficiency and high purity of the obtained product, and can be used for large-scale production of paclitaxel.
Owner:SUN YAT SEN UNIV
A kind of method that utilizes apple branch to prepare phloridzin
ActiveCN105777822BGood choiceHigh puritySugar derivativesSugar derivatives preparationTwigPromotion effect
The invention discloses a method for preparing high-purity phloridzin by virtue of apple tree branches. The method comprises the steps of raw material pretreatment, supercritical CO2-enzyme extraction, centrifugal partition chromatography purification and the like, and thus, the high-purity phloridzin is finally obtained. The method is applicable to preparation of the high-purity phloridzin with the apple tree branches as raw materials. According to the method, a supercritical CO2 extraction technology is combined with an enzyme-assisted extraction technology, and an enzymolysis process is implemented in supercritical CO2, so that enzymolysis effects and promotion effects on phloridzin extraction are greatly improved; in addition, supercritical CO2 extraction is high in selectivity, an entrainer is not required, and the product is easy to separate and high in purity; the extraction rate of the phloridzin is effectively increased, the high-purity phloridzin is obtained, the separation and purification time is saved, the yield is high, preparation procedures are simplified, and the method is a safe and efficient apple phloridzin extraction process, and has broad application prospect.
Owner:ZHENGZHOU FRUIT RES INST CHINESE ACADEMY OF AGRI SCI
Synthesizing method of gamma-aminopropyl triethoxysilane
InactiveCN101768180BImprove conversion rateImprove solubilitySilicon organic compoundsTriethoxysilaneDistillation
The invention discloses a synthesizing method of gamma-aminopropyl triethoxysilane, comprising the following steps of: (1) adding a toluene solution dissolved with a catalyst and the gamma-aminopropyl triethoxysilane to a reaction still under the condition of vacuum, adding ammonia in a stirring state, and increasing the temperature of materials inside the reaction still to 60-70 DEG C to react for 4-7 hours at the reaction pressure of 0.5-3 MPa; (2) after amination reaction is finished, filtering, separating and removing side products, and adding filter liquor to a distilling still; (3) after the top temperature of the distilling still is more than or equal to 102 DEG C, reducing the temperature of the materials inside the distilling still to be less than or equal to 80 DEG C, and carrying out reduced pressure distillation at vacuum degree of more than or equal to 0.099 MPa so as to obtain the gamma-aminopropyl triethoxysilane after the reduced pressure distillation. The invention enhances the purity of synthesized products by more than 99 percent (GC) and expands the application fields to industries, i.e. electronic and electric products, high-performance catalyst preparation, nanometer materials, superfine dispersing materials, and the like which have higher requirements for product purity.
Owner:青岛惠国新材料科技有限公司
Method for preparing salvianolic acid B through separation by means of flash chromatography
InactiveCN102532077BEasy to separateEasy extractionOrganic chemistryChromatographic separationMethanol water
Owner:SUN YAT SEN UNIV +1
Method for removing proteins and pigments in ganoderma lucidum crude polysaccharide
ActiveCN101724088BShorten separation and purification timeSimple purification processOrganic solventElution
The invention discloses a method for removing proteins and pigments in ganoderma lucidum crude polysaccharide, which comprises the following steps of: preparing the ganoderma lucidum crude polysaccharide into 10-30mg / m1 aqueous solution; centrifuging the aqueous solution to take a liquid supernatant; sampling the liquid supernatant; adding the liquid supernatant into a radial flow chromatographiccolumn filled with an anion exchanger; then eluting the chromatographic column with pure water to control a sampling flow velocity to 2-10ml / min and an elution flow velocity to 10-50ml / min; collecting an eluent until no saccharide is detected in the eluent; combining the collected eluent; and carrying out post-treatment to obtain a pure product of the ganoderma lucidum polysaccharide. The method has the advantages that: the proteins and the pigments in the ganoderma lucidum crude polysaccharide are removed simultaneously so as to greatly shorten separation and purification time of the polysaccharide, be favorable for reserving the polysaccharide activity maximally and improve the purification efficiency of the ganoderma lucidum crude polysaccharide; organic solvents are not used in an operation process, conditions are mild, subsequent treatment is convenient, and environmental pollution is lightened; and the iron exchange resins have low cost and can be recycled so as to effectively reduce the production cost.
Owner:ZHEJIANG UNIV OF TECH
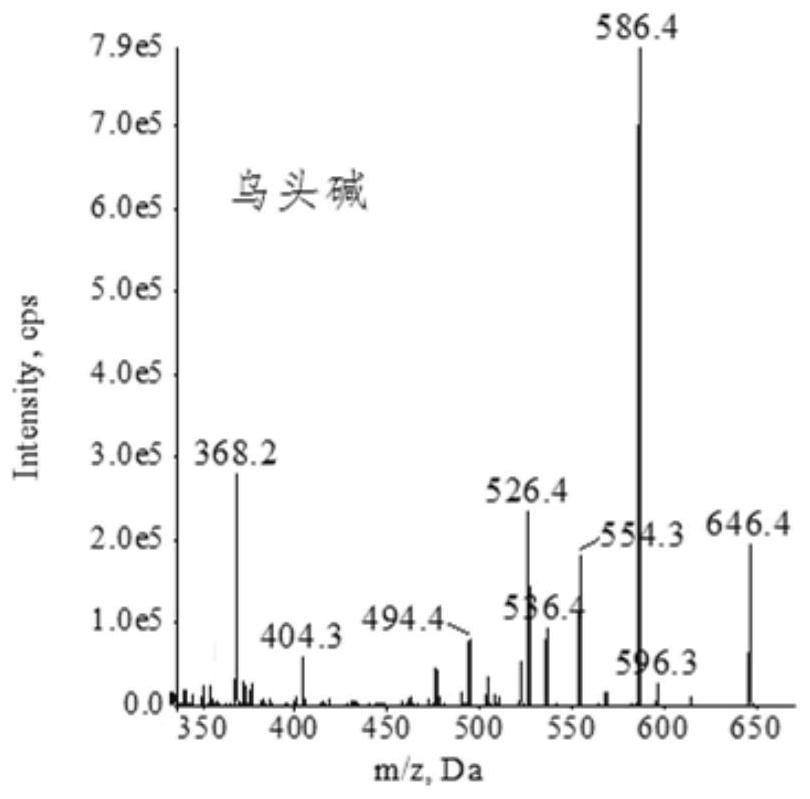
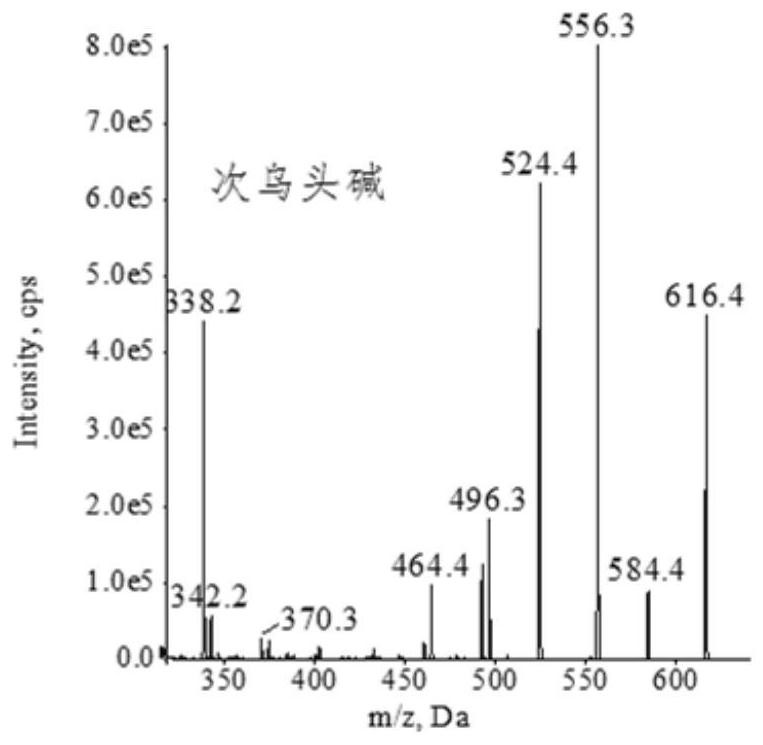
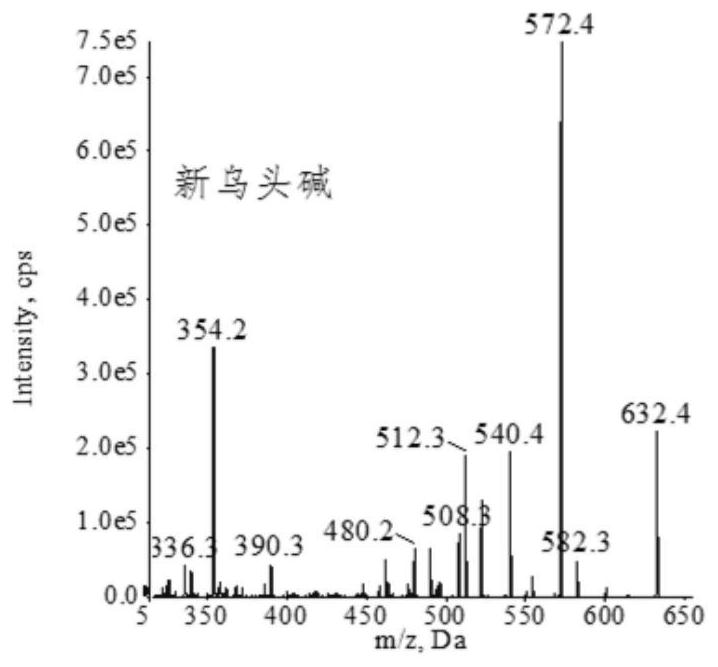
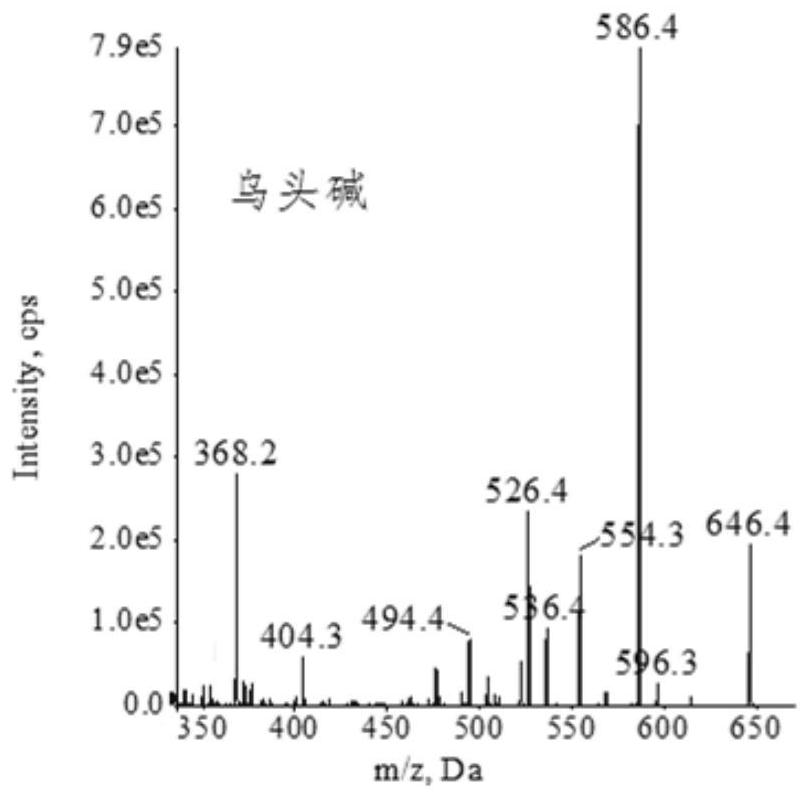
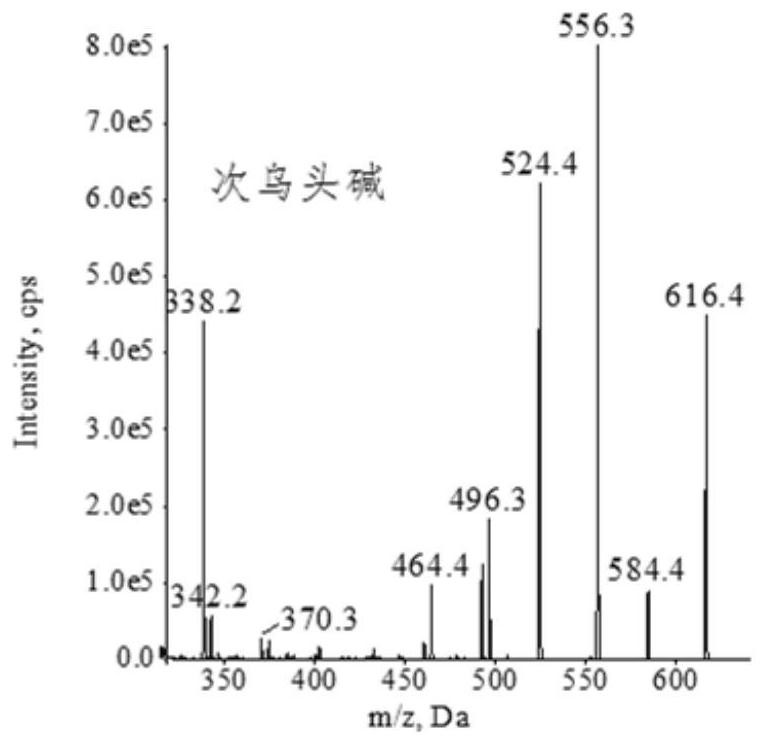
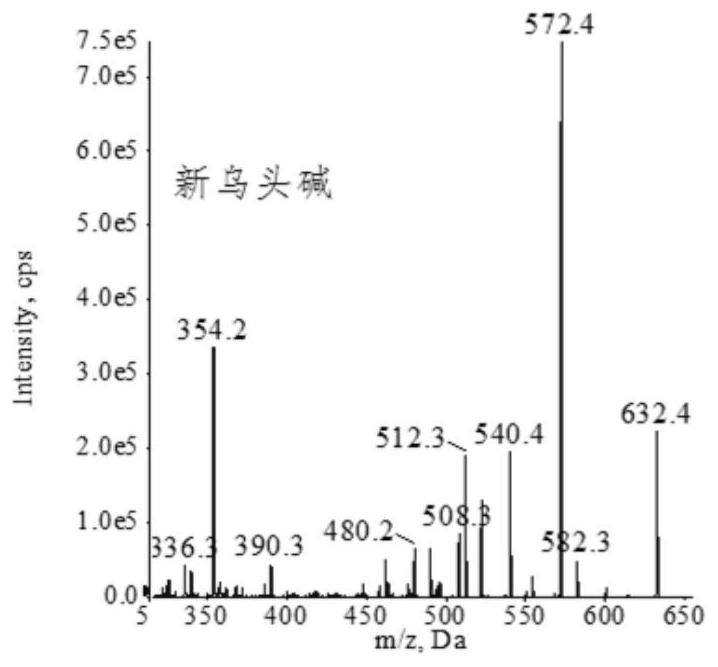
Extraction, separation and purification method of aconitum alkaloid
ActiveCN112694442AHigh extraction rateFast separationOrganic chemistryChromatographic separationPurification methods
The invention discloses an extraction, separation and purification method of aconitum alkaloid, which belongs to the technical field of alkaloid extraction. The method comprises the following steps of by taking plant tubers containing aconitum alkaloid as raw materials, crushing, sieving, performing ultrasonic-assisted extraction by adopting an acidified ethanol solution, and removing a solvent through deesterification, alkalization and reduced pressure distillation to obtain crude alkaloid, then separating by adopting high-speed counter-current chromatography, finally preparing high-content aconitum alkaloid monomers by adopting reversed-phase high-performance liquid-phase semi-preparative chromatography, carrying out ultraviolet online detection, collecting a target object, and carrying out freeze drying to obtain aconitine, aconitine, neoaconitine and aconitine yunnanense with purity greater than 98.0%. By adopting the preparation method disclosed by the invention, the separated product is high in yield, high in purity, short in process, low in cost and easy to industrialize.
Owner:NINGBO MUNICIPAL CENT FOR DISEASE CONTROL & PREVENTION
A kind of preparation method of Cimicifuga saponins
The invention provides a method for preparing total saponins of rhizoma cimicifugae through separation and purification by virtue of a micro emulsion-phase interface extraction technique. The method can achieve the purpose of realizing industrial mass production by virtue of the micro emulsion extraction technique, and the method can save time and reduce the use of an organic solvent; and in addition, the clinical efficacy of the total saponins of rhizoma cimicifugae prepared by the method is better than that of total saponins of rhizoma cimicifugae obtained by a conventional separation and purification method.
Owner:SHANDONG LUYE PHARMA CO LTD
A method for separation and purification of fmd-inactivated virus antigens using simulated fluidized bed
ActiveCN109836479BImprove continuityEasy to operateVirus peptidesPeptide preparation methodsFluidized bedElution
The invention relates to a method for separating and purifying an FMD inactivated virus antigen by applying a simulated flow bed, wherein the method comprises the following steps: balancing a chromatographic column of the simulated flow bed with a balanced buffer solution; loading an FMD inactivated virus antigen sample into the chromatographic column of the simulated flow bed, during sample loading, simultaneously carrying out antigen sample impurity cleaning and sample desorption elution as well as chromatographic filler cleaning and regeneration and balanced liquid balancing on the chromatographic column, and continuously desorbing and eluting the collected and purified sample in the process. The simulated flow bed is applied to separation and purification of the FMD inactivated virus antigen for the first time, the continuous saturated sample loading separation operation can be realized and the sample loading amount is greatly improved; moreover, through the continuous separation and purification via multiple monomer columns, the degree of continuity is high, the separation and purification time is greatly shortened, the preparation efficiency is improved and the operation costis reduced. Moreover, the rigid filler is selected specifically, the loading capacity of the filler namely the pressure resistance is greatly improved, the use amount of the chromatographic filler isreduced, and the service life of the chromatographic column in the simulated flow bed is prolonged.
Owner:SHANGHAI SHEN LIAN BIOMEDICAL CORP
Method for separating and purifying oligodendrocyte precursor cells
InactiveCN101735983BAvoid damageIncrease acquisition rateNervous system cellsSingle cell suspensionPolylysine
The invention discloses a method for separating and purifying oligodendrocyte precursor cells. The method comprises: digesting cerebral cortex of neonatal rats and part of white matter with trypsin and DNA enzyme; blowing and beating the obtained product to be a single-cell suspension; inoculating the single-cell suspension into a culture flask coated with polylysine in advance by use of a mixed-cell culture medium; performing culture for 3 to 5 days; replacing the culture flask with an oligodendrocyte precursor cell proliferation culture medium when the fusion of bottom-layer cells reaches 65 to 75 percent; performing culture for 3 to 5 days; replacing the oligodendrocyte precursor cell proliferation culture medium with an oligodendrocyte precursor cell separation culture medium; digesting the obtained product at 37 DEG C; isolating oligodendrocyte precursor cells from mixed cells; blowing and beating the obtained product to be the single-cell suspension; inoculating the single-cell suspension into the culture flask coated with polylysine in advance by use of an oligodendrocyte precursor cell inoculation culture medium; replacing the culture flask with an oligodendrocyte precursor cell purification culture medium after the cells adhere to a wall; performing culture for 2 to 4 days; and obtaining adherence cells, namely the oligodendrocyte precursor cells. The method can obtain a large number of high-purity good-activity oligodendrocyte precursor cells conveniently, rapidly, economically and efficiently.
Owner:ARMY MEDICAL UNIV
Separation and purification method of c-di-GMP (cyclic diguanylate)
InactiveCN102443031BMeet the needs of scientific research workImprove recycling efficiencySugar derivativesSugar derivatives preparationPurification methodsFreeze-drying
The invention discloses a separation and purification method of c-di-GMP (cyclic diguanylate), comprising the following steps of: directly sampling a c-di-GMP crude product solution on a fast protein liquid chromatography equipped with an ion exchange chromatographic column; carrying out gradient elution by using a buffer solution with pH of 8.0; carrying out detection under 254 nm by an ultraviolet detector; collecting an eluent in the peak position; confirming a structure by using a mass spectrum; and freeze-drying the eluent with target compounds, thereby obtaining the solid c-di-GMP. The c-di-GMP prepared according to the method provided by the invention has the purity of over 95% through reversed-phase high performance liquid chromatography detection, and realizes c-di-GMP separation and purification from the c-di-GMP crude product solution in one step; moreover, the method provided by the invention has simple technology, short period and nonuse of toxic organic solvents, and is a novel method with high efficiency, environment friendliness and suitability for industrialized production.
Owner:SHANDONG UNIV
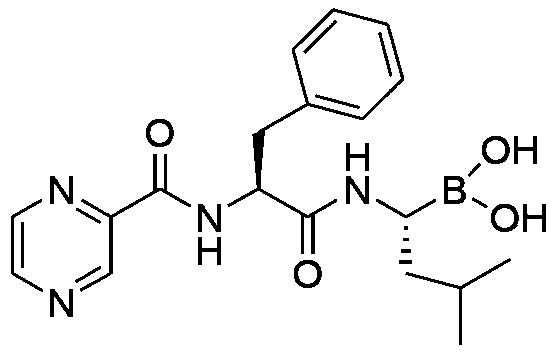
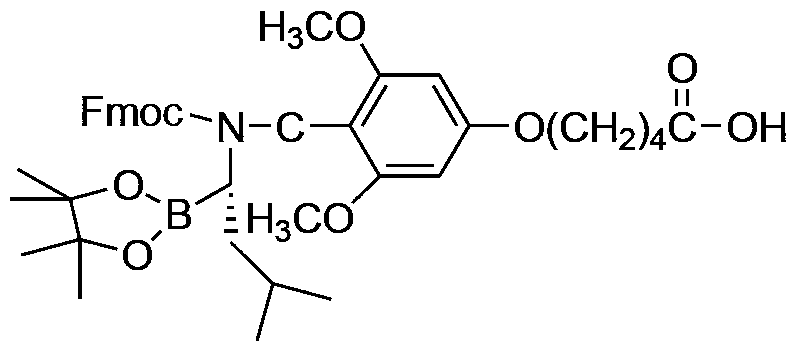
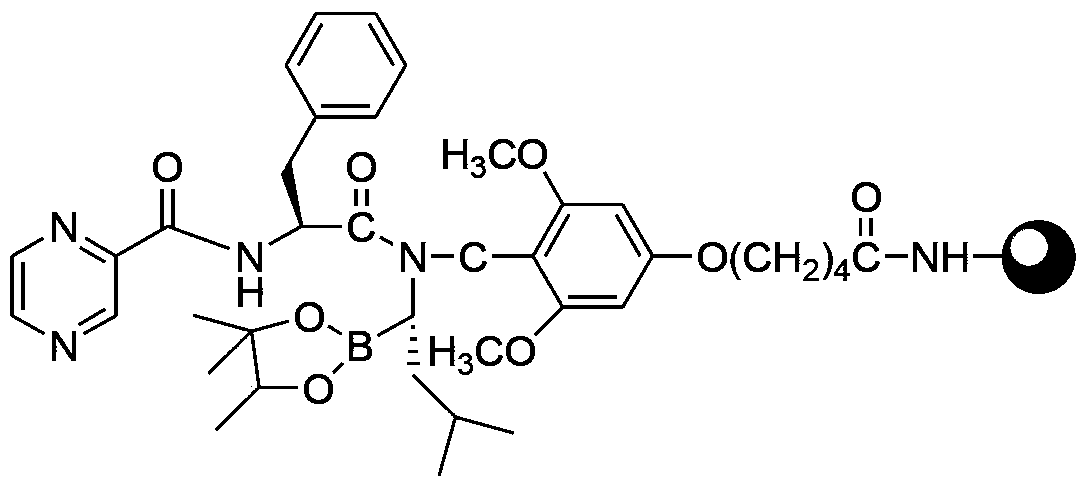
A kind of preparation method of bortezomib
InactiveCN104211758BThorough responseAvoid post-processing operationsPeptide preparation methodsPyrazineBoronic acid
The invention provides a preparation method of bortezomib. The preparation method comprises the following steps: step a, taking pinacol-1-amino-3-methylbutane-1-borate and 5-(4-formyl-3,5-dimethoxylphenoxyl)pentanoic acid as the primary raw materials, subjecting the raw materials to condensation reactions, reduction reactions, and Fmoc-protection reactions so as to obtain an intermediate, which is represented in the description; step b, taking a resin as the solid carrier, sequentially coupling the intermediate obtained in the step a, Fmoc-L-phenylalanine, and pyrazine-2-carboxylic acid so as to obtain a resin compound, which is represented in the description; step c, cutting the resin compound obtained in the step b so as to obtain bortezomib borate; and step d, hydrolyzing bortezomib borate obtained in the step c so as to obtain the finished product. The preparation method of bortezomib has the advantages of simple procedure and equipment, easy operation, and greatly-improved yield.
Owner:HYBIO PHARMA
A kind of corn pollen separation and purification method
ActiveCN104970279BShorten separation and purification timeHigh extraction rateCosmetic preparationsFood freezingFiltrationBiology
The invention provides a process method for separating and purifying maize pollen from maize anther. Maize male spikes (the male spikes are extracted and are close to dusting period) are extracted cross the ridge, and anther on the male spikes is rubbed down. The maize anther comprises two parts: pollen capsule walls and pollen wrapped thereby. The pollen and the pollen capsule walls are initially separated by water-soaking and filtering methods, the tissues of the filter residues (the pollen capsule wall) are titrated, and the titrated pollen capsule walls are further soaked in water and filtered so as to further separate the pollen from the pollen capsule walls. The filtrates of filtration twice are mixed together to obtain relatively pure pollen, and the filtrate is filtered, so that the effect of eliminating fine impurities and purifying the maize pollen can be achieved, and the filtrate is lyophilized to obtain pure maize pollen. The method is simple, feasible and high in efficiency, the nutrients of the maize pollen are not destroyed and the maize pollen is not damaged, and the method is suitable for scaled production.
Owner:SICHUAN UNIV
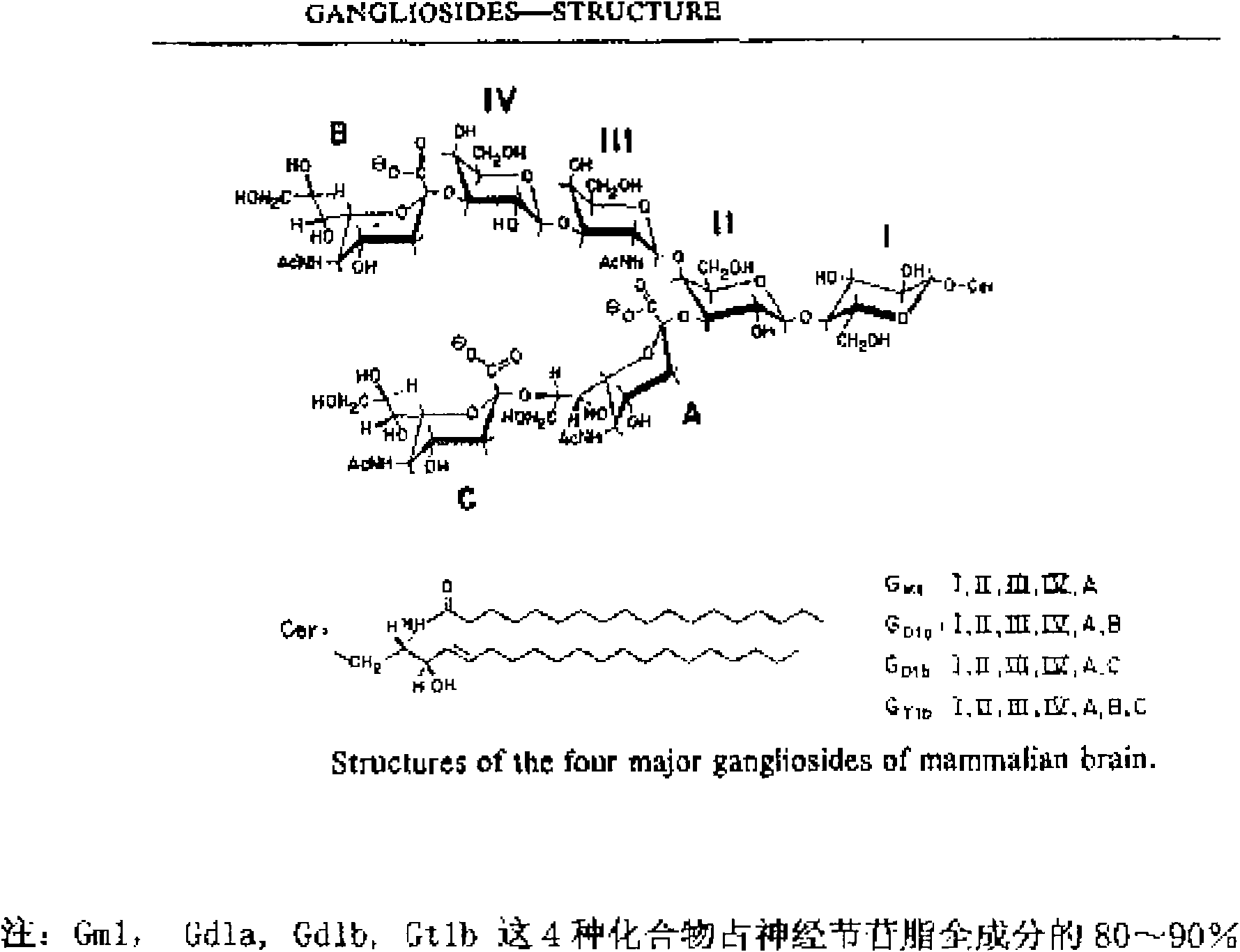
Industrial production process for fast separating and purifying ganglioside by using chromatography media having special identification function
ActiveCN101838295BGuaranteed purityShort separation and purification cycleIon-exchange process apparatusSugar derivativesCentrifugationAnimal brain
The invention provides an industrial production process for fast separating and purifying ganglioside by using chromatography media having a special identification function. The process comprises three steps of static adsorption, elution, and concentration and drying, wherein the step of static adsorption comprises the steps of: adding extracting solution into animal brain tissue for homogenation, centrifugation and demixing, directly adding supernatant liquid subjected to the demixing into the chromatography media having a special identification function, stirring for absorption for 1 to 2 hours and fully stirring and mixing the chromatography media having the special identification function and the extracting solution; the step of elution comprises the step of directly adding the chromatography media having the special identification function and absorbing the ganglioside into elution liquid for elution for 1 to 2 hours; and the step of concentration and drying comprises the steps of: concentrating the elution liquid and then refrigerating and drying the concentrated elution liquid in the environment of -70 to 20 DEG C to obtain the ganglioside product. In the process, the chromatography media and the extracting solution are fully stirred and mixed by using the methods of static absorption and elution to minimize the loss of the ganglioside in the extracting process; compared with the conventional macroporous resin chromatography, the process has the advantages that: the yield is increased by 15 to 20 percent, the separation and purification time is shortened by 10 to 38 times; moreover, the process is simple to operate and favorable for industrial production.
Owner:李桂凤
Radial flow chromatographic separation and purification method for biological polysaccharide
InactiveCN101914165BHigh purityLow pigment contentIon-exchange process apparatusIon-exchanger regenerationChromatographic separationPurification methods
The invention relates to a radial flow chromatographic separation and purification method for biological polysaccharide, which comprises the following steps of: centrifuging a medical fungi liquid fermentation product or a mycelium (fruiting body) enzymolysis product and collecting supernatant or collecting supernatant which comprises polysaccharide and is extracted from plants, animals, bacteriaand yeast; directly loading the supernatant on a macroporous resin radial chromatographic column of 500ml at the flow rate of 60 to 120ml per minute; eluting the macroporous resin radial chromatographic column by using distilled water or deionized water at the flow rate of 50 to 200ml per minute; and collecting 500 to 800ml of eluent, namely solution containing purified polysaccharide. The polysaccharide is directly separated and purified from the enzymolysis supernatant and the fermentation supernatant of the medical fungi mycelium and the supernatant extracted from the plants, animals, bacteria and yeast by a one-step method; and the biological polysaccharide separation and purification method has the advantages of simple process, convenient operation, no use of an organic solvent, highproduction efficiency and obvious purification effect.
Owner:HARBIN INST OF TECH AT WEIHAI
Rapid separation and purification method of cordyceps militaris fruit body water-soluble peptide polysaccharide
InactiveCN101200491BAvoid collectingSimple processPeptide preparation methodsOn columnPurification methods
The invention discloses a rapid separation and purification method of the water soluble peptide polysaccharide from Cordyceps Militaris and belongs to the extraction technology field of the bioactive substances. The method comprises drying and smashing the Cordyceps Militaris, obtaining the component CF1 by extracting the ultrapure water, obtaining the component CF1-1 by the separation from the molecular sieve, obtaining the component CF1-1-1 and CF1-1-2 by separation and purification from the ionic exchange column, and obtaining the CF1-1-1-1, CF1-1-2-1 and CF1-1-2-2 by separation from the affinity chromatography column. By adopting the purification method of smashing the Cordyceps Militaris and direct introduction of the water extract on column, the invention greatly promotes the efficiency and decreases the cost, in addition, the entire separation and purification process does not need the organic solvent, thereby really achieving the purpose of green, rapidness and high efficiencyof the separation of the polysaccharide. The method can be widely applied in the separation and purification technology of the water soluble peptide polysaccharide from the Cordyceps Militaris in thefactory and the laboratory.
Owner:ZHEJIANG ACADEMY OF AGRICULTURE SCIENCES
A method for extracting, separating and purifying aconitum alkaloids
ActiveCN112694442BHigh extraction rateFast separationOrganic chemistryFluid phasePurification methods
The invention discloses a method for extracting, separating and purifying aconitum alkaloids, belonging to the technical field of alkaloid extraction. The raw material is root tuber containing aconitum alkaloids, crushed and sieved, extracted with acidified ethanol solution ultrasonically, deesterified and alkalized, and distilled under reduced pressure to remove the solvent to obtain crude alkaloids; then separated by high-speed countercurrent chromatography, and finally Aconitine, hypoaconitine, Neoaconitine and Dianaconitine. By adopting the preparation method disclosed by the invention, the separated product has high yield, high purity, short process, low cost and easy industrialization.
Owner:NINGBO MUNICIPAL CENT FOR DISEASE CONTROL & PREVENTION
Preparation method of NGF for cobra venom injection
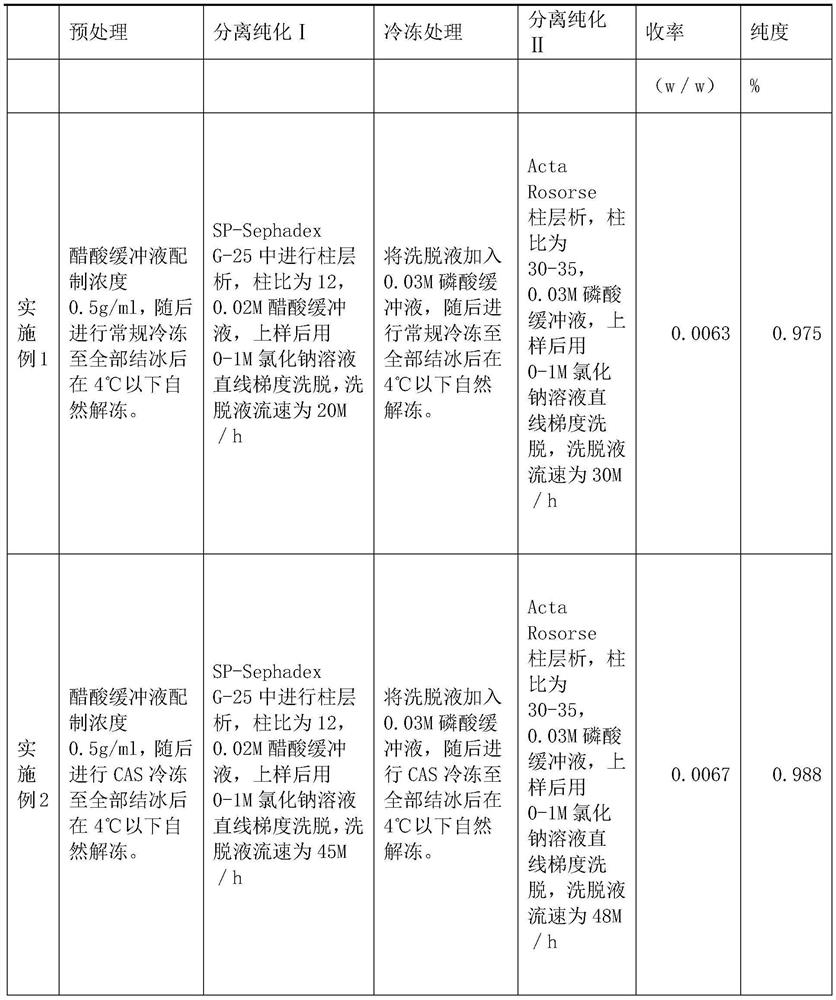
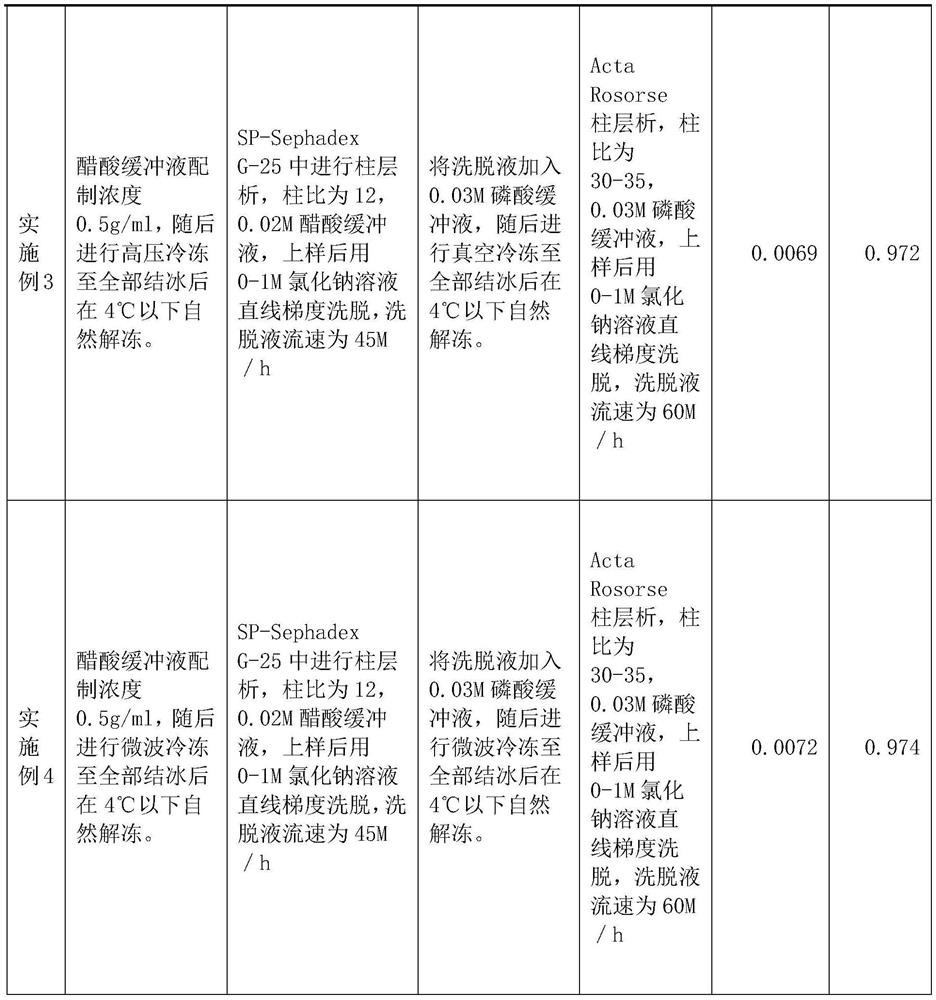
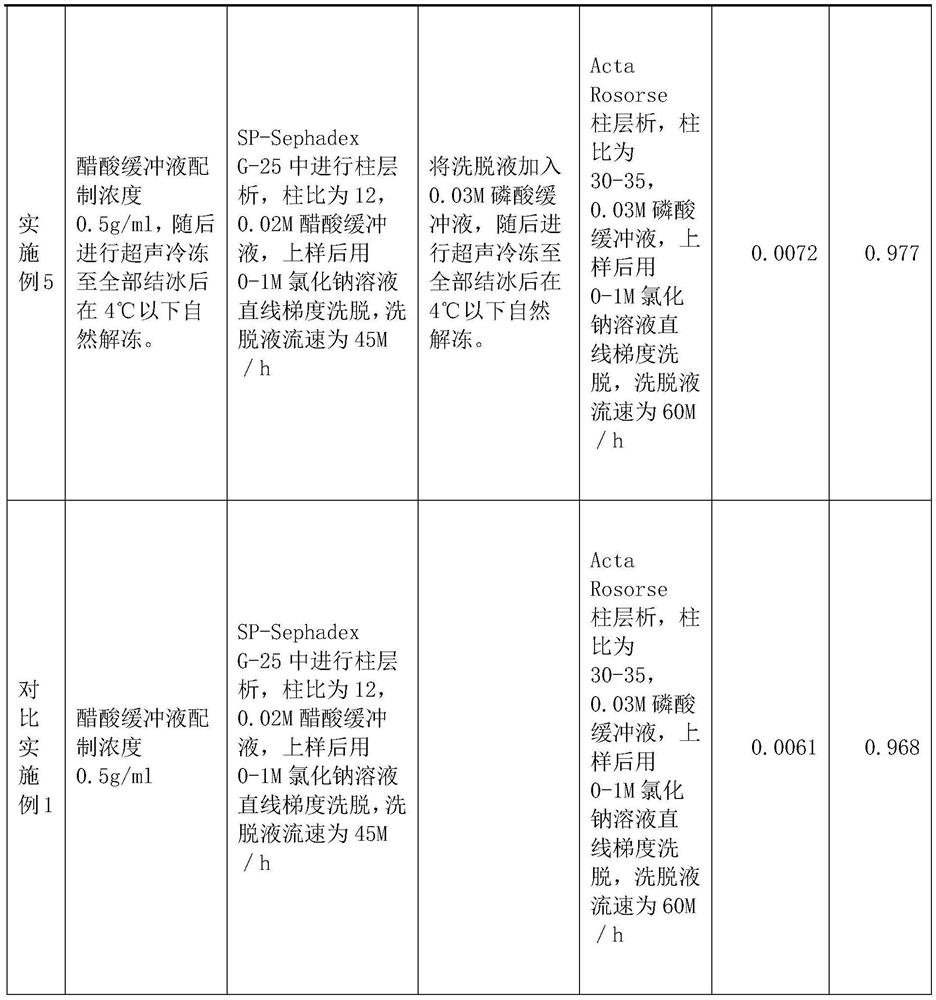
PendingCN113416242ASimple manufacturing methodLittle loss of activityPeptide preparation methodsAnimals/human peptidesCobra venomSephadex
The invention relates to a preparation method of NGF for cobra venom injection, wherein crude cobra venom is subjected to gel chromatography twice, gel chromatography I is SP-Sephadex G-25 column chromatography, gel chromatography II is Acta Rose column chromatography, and snake venom added with a buffer solution before gel chromatography I and crude snake venom nerve growth factor liquid after gel chromatography I are subjected to freezing treatment. The cobra venom nerve growth factor provided by the invention is simple and feasible in preparation method, can be used for industrial large-scale production, and is small in activity loss, and the activity loss of the cobra venom NGF is less than 10%; the conventional limitation of protein separation can be broken through, and the crude snake venom liquid with higher initial concentration can also be used for effective separation; the method is high in production efficiency, reasonable in cost and particularly suitable for industrial large-scale production; the yield is high and exceeds 0.6%, and even can reach 0.7% or above.
Owner:云南龙凤谷生物药业有限公司 +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com