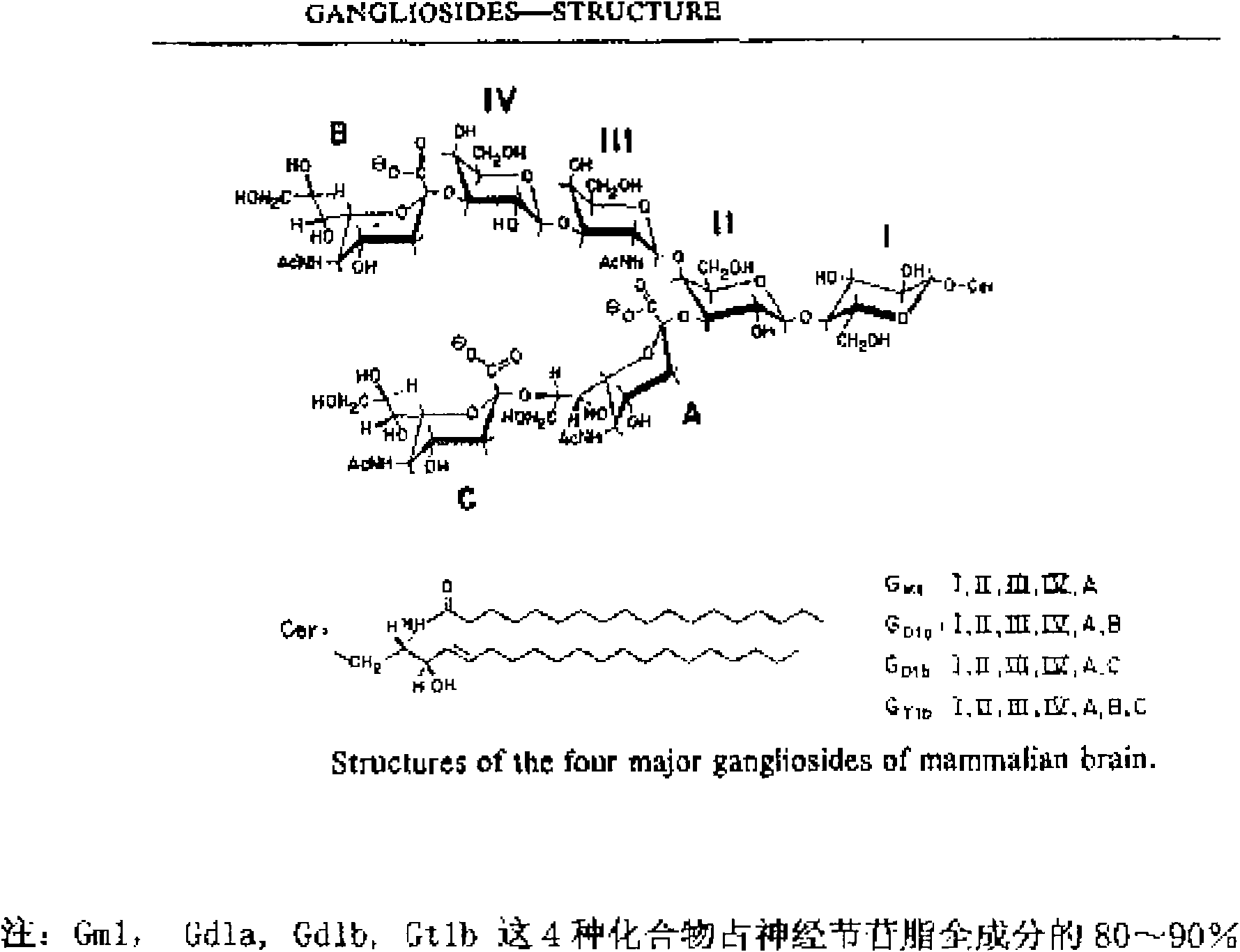
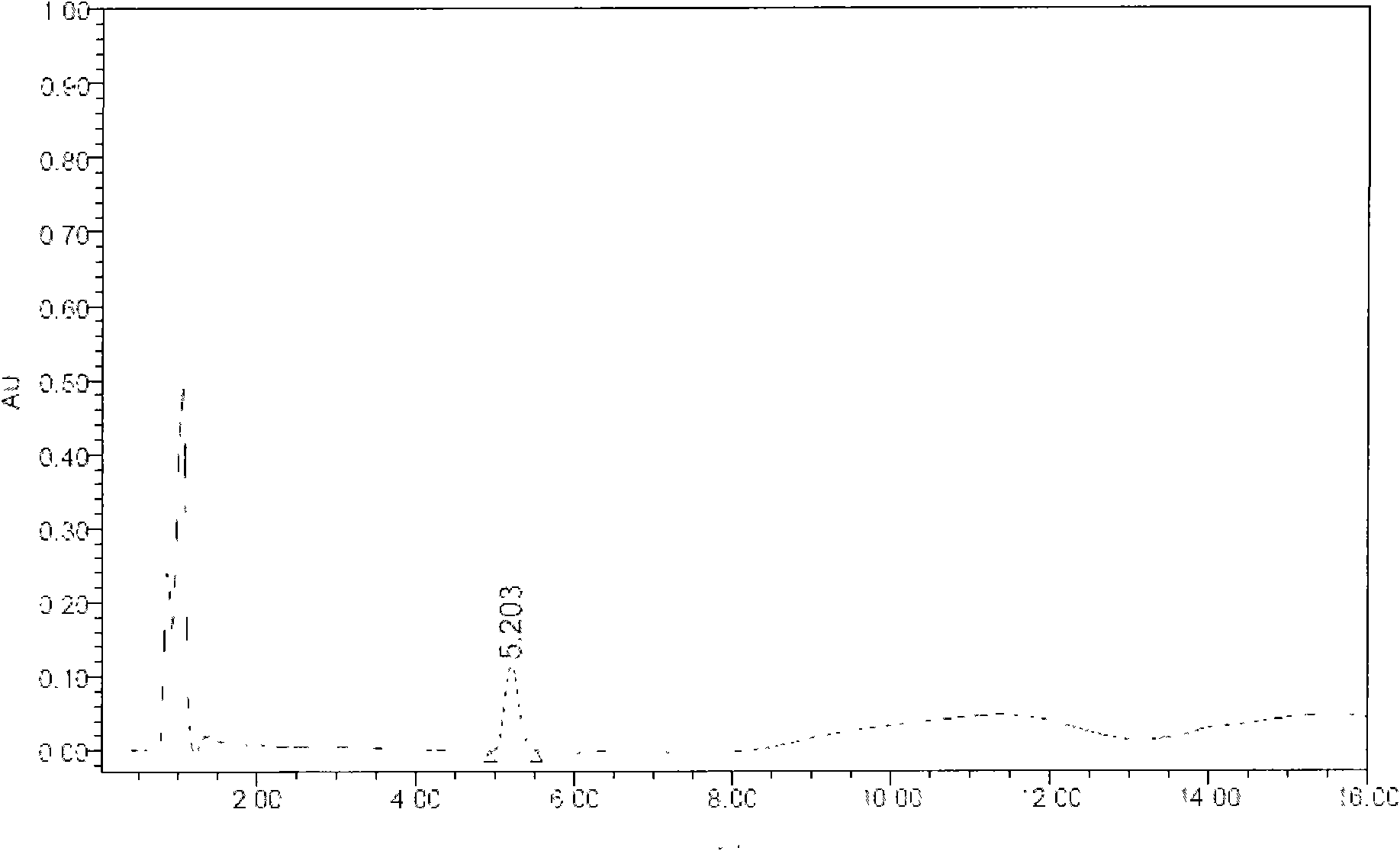
Industrial production process for fast separating and purifying ganglioside by using chromatography media having special identification function
A technology of gangliosides and chromatographic media, which is applied in the field of industrial production technology, can solve the problems of lack of treatment measures and high morbidity, and achieve the effects of shortening the separation and purification time, simple operation, and increasing yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Embodiment 1: Take pig fresh brain tissue as an example for illustration.
[0030] 1) Development of chromatographic medium with specific recognition function:
[0031] Pretreatment of medical stone: add medical stone with a particle size of 20-60 mesh into 1mol / L hydrochloric acid, the mass ratio of medical stone to hydrochloric acid solution is 1:12; soaking time is 30 hours; remove organic matter and other impurities, After soaking, wash the medical stone with distilled water until it is neutral, and dry it for later use;
[0032] Preparation of chromatographic medium with specific recognition function: first add chitosan with acetylation degree of 75% to acetic acid solution with a concentration of 6%, and prepare 60ml of chitosan solution with a concentration of 0.3%; Add 20g of medical stone into the chitosan solution to make a paste, make it fully infiltrated, put the paste in a microwave oven, heat and dry, grind it through a 100-mesh sieve, and then get a chro...
Embodiment 2
[0039] Embodiment 2: Take fresh bovine brain tissue as an example for illustration.
[0040] 1) Development of chromatographic medium with specific recognition function:
[0041]Pretreatment of medical stone: add medical stone with a particle size of 20-60 mesh into 2.5mol / L hydrochloric acid, the mass ratio of medical stone to hydrochloric acid solution is 1:15; soaking time is 38 hours; remove organic matter and other impurities , after soaking, wash the medical stone with distilled water until it is neutral, and dry it for later use;
[0042] Preparation of chromatographic medium with specific recognition function: first add chitosan with an acetylation degree of 90% to an acetic acid solution with a concentration of 8%, and prepare 120ml of a chitosan solution with a concentration of 0.5%; Add 50g of medical stone into the chitosan solution to make a paste, fully infiltrate it, put the paste in a microwave oven, heat and dry, grind and pass through a 120-mesh sieve to obt...
Embodiment 3
[0049] Embodiment 3: Take fresh sheep brain tissue as an example for illustration.
[0050] 1) Development of chromatographic medium with specific recognition function:
[0051] Pretreatment of medical stone: add medical stone with a particle size of 20-60 mesh into 0.5mol / L hydrochloric acid, the mass ratio of medical stone to hydrochloric acid solution is 1:10; soaking time is 20 hours; remove organic matter and other impurities , after soaking, wash the medical stone with distilled water until it is neutral, and dry it for later use;
[0052] Preparation of chromatographic medium with specific recognition function: first add chitosan with 60% acetylation to 1% acetic acid solution to prepare 180ml of chitosan solution with 0.1% concentration; Add 80g of medical stone into the chitosan solution to make a paste, fully infiltrate it, put the paste in a microwave oven, heat and dry, and grind it through a 90 mesh sieve to obtain a chromatographic medium with a specific recogni...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com