New mutant disease-causing gene of Alport syndrome, encoded protein and application thereof
A technology of gene coding and syndrome, applied in the field of mutated disease-causing genes, can solve the problems of children's kidneys not being observed and the difficulty of rapid diagnosis, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Example 1: Sample acquisition
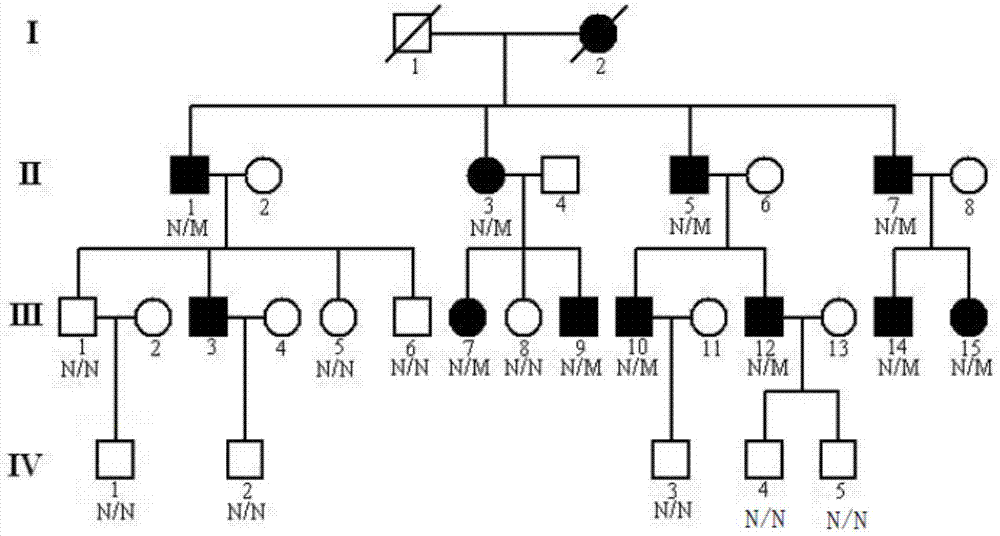
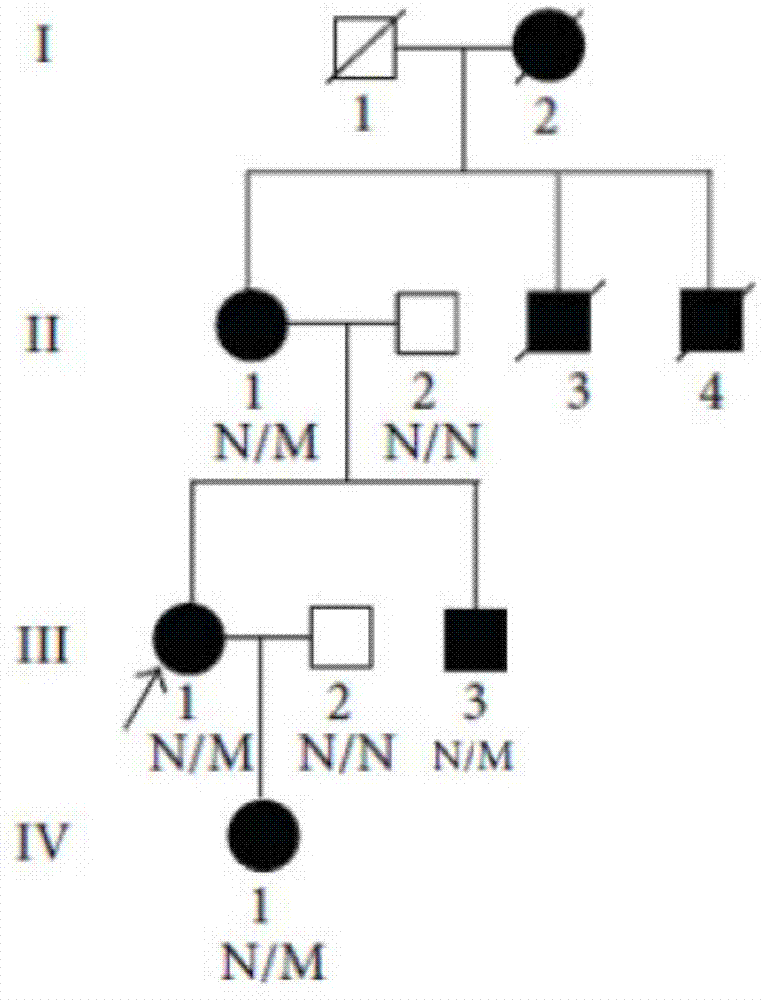
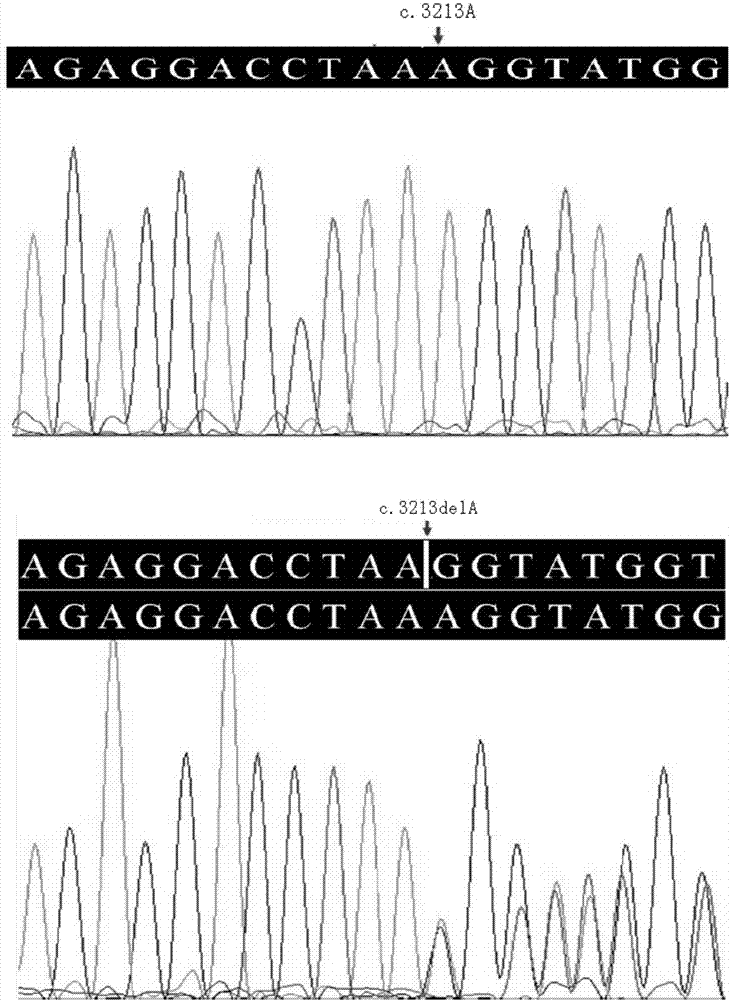
[0047] The inventor has collected two ATS families in China in recent years. Family 1 has 4 generations of 30 Han family members ( figure 1 ), showing autosomal dominant inheritance, family 2 has 4 generations of 10 Han family members ( figure 2 ), showing X-chromosomal dominant inheritance, all family members in family 1 and family 2 underwent routine urine and renal function tests. Both the proband of family 1 (III:12, case 1) and the proband of family 2 (III:1, case 2) were found to have total and segmental sclerosis and mesangial expansion by renal biopsy, and glomerular basement membrane was found by electron microscopy (GBMS) showed irregular thickening and splitting, based on clinical and biochemical indicators and imaging evidence, combined with the familial cases we collected, it suggested that ATS was more likely. 10 patient samples were selected in family 1 (II:1, II:3, II:5, II:7, III:7, III:9, III:10, III:12, III:14, and I...
Embodiment 2
[0049] Embodiment 2: sample DNA preparation
[0050] OMEGA Blood DNA Midi Kit whole blood DNA extraction kit was used to extract DNA from peripheral blood samples, and the extraction steps were as follows:
[0051] (1) Take 2ml whole blood sample, add 150ul OB Protease, 2.1ml Buffer BL and 20ul RNase A, vortex at maximum speed for 1 minute, and mix thoroughly.
[0052] (2) 65°C water bath for 15-20 minutes, and vortex 5 times during the water bath.
[0053] (3) Add 2.2ml of absolute ethanol, vortex at maximum speed for 30 seconds, and mix thoroughly.
[0054] (4) Transfer 3.5ml of the lysate into a 15ml centrifuge tube with a filter column, centrifuge at 4000 rpm for 5 minutes, take out the filter column, pour off the filtered liquid, and put it back into the filter column.
[0055] (5) Add the remaining lysate from step 3 into a 15ml centrifuge tube with a filter column, centrifuge at 4000 rpm for 5 minutes, take out the filter column, pour off the filter liquid, and put it...
Embodiment 3
[0063] Example 3: Exon capture and sequencing
[0064] The inventor used Agilent SureSelect Human All Exon Capture Kit (Agilent SureSelect Human All Exon Kit) in combination with Solexa high-throughput sequencing technology to sequence the exome sequence of the sample of Example 1 selected, as follows:
[0065] 1) Genomic DNA was randomly broken into fragments of about 150-200bp, and then adapters were connected to both ends of the fragments to prepare a hybrid library (see the Illumina / Solexa standard library construction instructions provided by http: / / www.illumina.com / ) .
[0066] 2) After the library is purified, it undergoes ligation-mediated PCR (ligation-mediated PCR (LM-PCR)) linear amplification and SeqCap EZ Oligo pool for hybridization enrichment, and then performs sequencing on the machine after linear amplification of LM-PCR . The sequencing platform is Illumina Hiseq2000, the read length is 90bp, and the average sequencing depth of each sample is at least 50×. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com