Preparation method of graphene-modified tin oxide lithium ion battery negative material
A lithium-ion battery and tin dioxide technology, applied in battery electrodes, secondary batteries, circuits, etc., can solve problems such as high operating environment requirements, difficult waste liquid treatment, and complex equipment, and achieve convenient operation, abundant raw materials, and distributed even tight effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
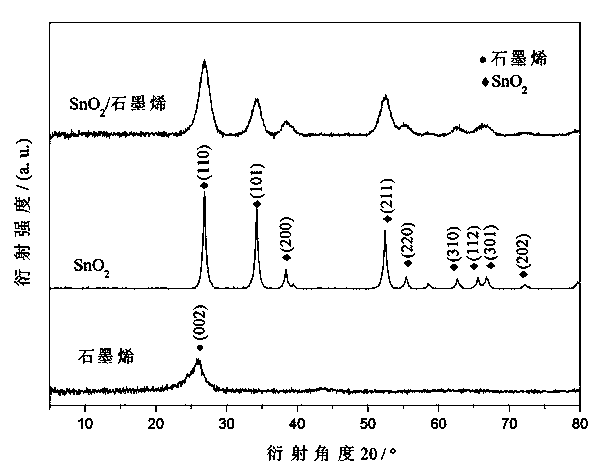
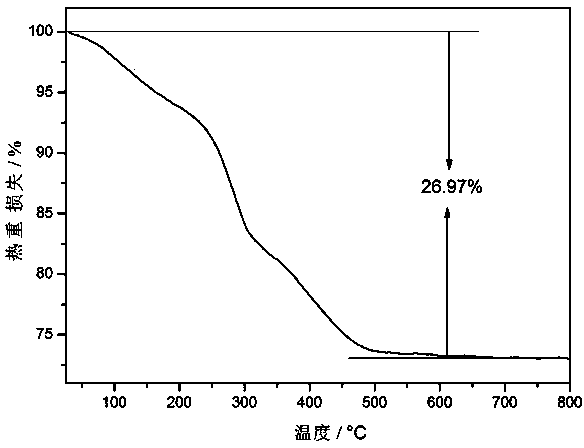
Image
Examples
Embodiment 1
[0028] A preparation method of a graphene-modified tin dioxide lithium-ion battery negative electrode material, comprising the steps of:
[0029] (1) Accurately weigh 1.0000g of metal tin and dissolve it in 20ml of dilute nitric acid with a concentration of 4M under ice bath and stirring conditions, and add 34ml of citric acid with a concentration of 0.5M under continuous stirring conditions to make divalent tin The molar ratio of ions to citric acid is 1:2, then adjust the pH of the solution to 7 with 20wt% ammonia water, and finally dilute it to 200 ml with deionized water to obtain a light yellow transparent Sn(OH) with a concentration of 0.042M 2 positive sol;
[0030] (2) Accurately weigh 0.1000g of graphene oxide with 150W ultrasound for 30min and disperse it in 500mL of water to obtain a graphene oxide negative sol with a pH of 7 and a concentration of 0.2mg / mL;
[0031] (3) Under stirring conditions, the Sn(OH) 2 The positive sol was dripped into the graphene oxide n...
Embodiment 2
[0035] A preparation method of a graphene-modified tin dioxide lithium-ion battery negative electrode material, comprising the steps of:
[0036] (1) Accurately weigh 1.0000g of metal tin and dissolve it in 20ml of dilute nitric acid with a concentration of 4M under ice bath and stirring conditions, and add 34ml of citric acid with a concentration of 0.5M under continuous stirring conditions to make divalent tin The molar ratio of ions to citric acid is 1:2, then adjust the pH of the solution to 7 with 20wt% ammonia water, and finally dilute to 200 ml with deionized water to obtain a concentration of 0.042M light yellow transparent Sn(OH) 2 positive sol;
[0037] (2) Accurately weigh 0.3000g of graphene oxide with 100W ultrasound for 60min and disperse it in 500mL of water to obtain a graphene oxide negative sol with a pH of 7 and a concentration of 0.6mg / mL;
[0038] (3) Under stirring conditions, the Sn(OH) 2 The positive sol was slowly dripped into the graphene oxide nega...
Embodiment 3
[0042] A preparation method of a graphene-modified tin dioxide lithium-ion battery negative electrode material, comprising the steps of:
[0043] (1) Accurately weigh 3.0000g of metal tin and dissolve it in 20ml of dilute nitric acid with a concentration of 4M under ice bath and stirring conditions, add 50ml of citric acid with a concentration of 1M under continuous stirring to make divalent tin ions and lemon The molar ratio of the acid is 1:2, then the pH of the solution is adjusted to 7 with 25wt% ammonia water, and finally diluted to 200 ml with deionized water, the available concentration is 0.126M light yellow transparent Sn(OH) 2 positive sol;
[0044] (2) Accurately weigh 0.1000g of graphene oxide with 100W ultrasound for 40min and disperse it in 500mL of water to obtain a graphene oxide negative sol with a pH of 7 and a concentration of 0.2mg / mL;
[0045] (3) Under stirring conditions, the Sn(OH) 2 The positive sol was dropped into the graphene oxide negative sol at...
PUM
| Property | Measurement | Unit |
|---|---|---|
| crystal size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com