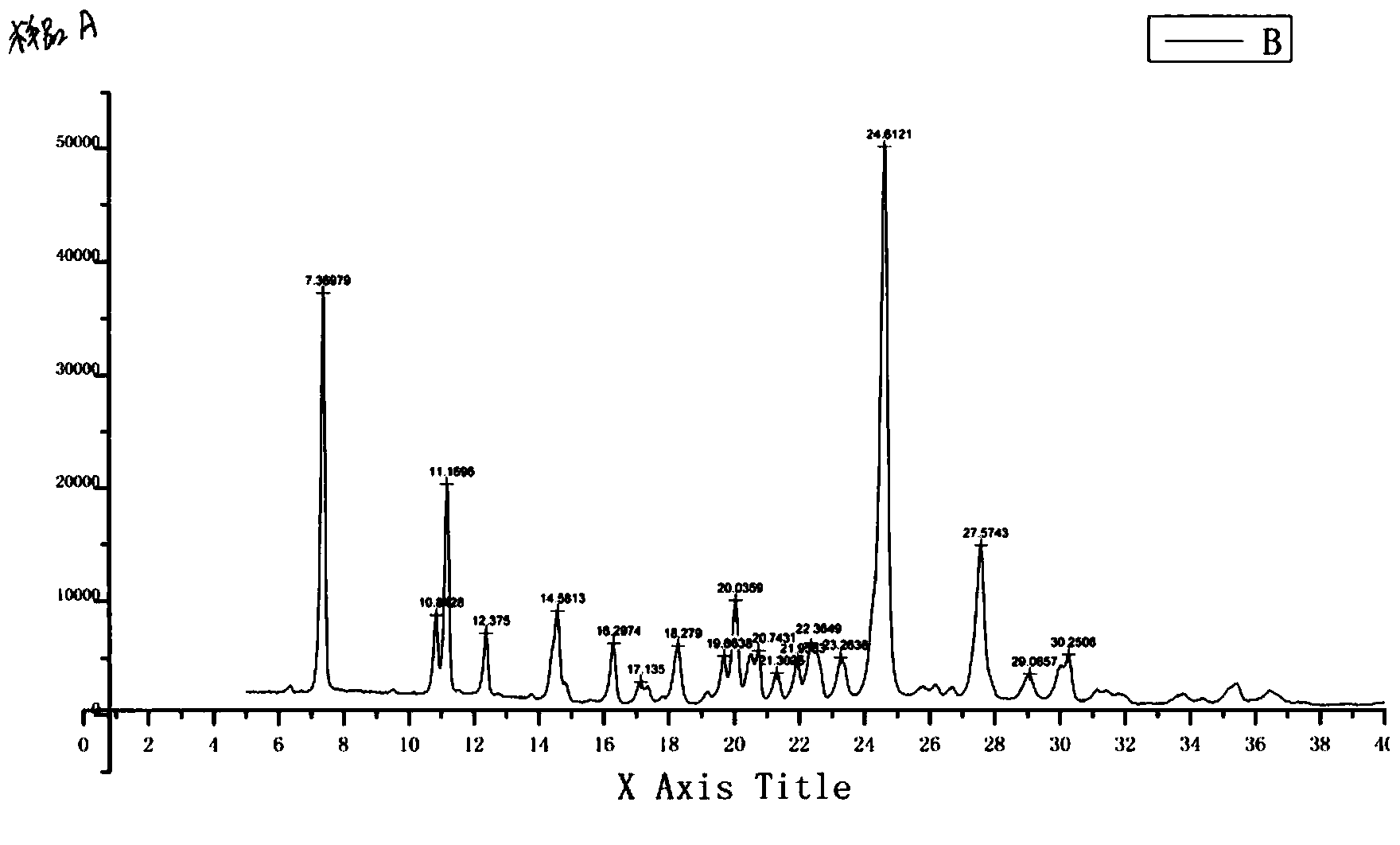
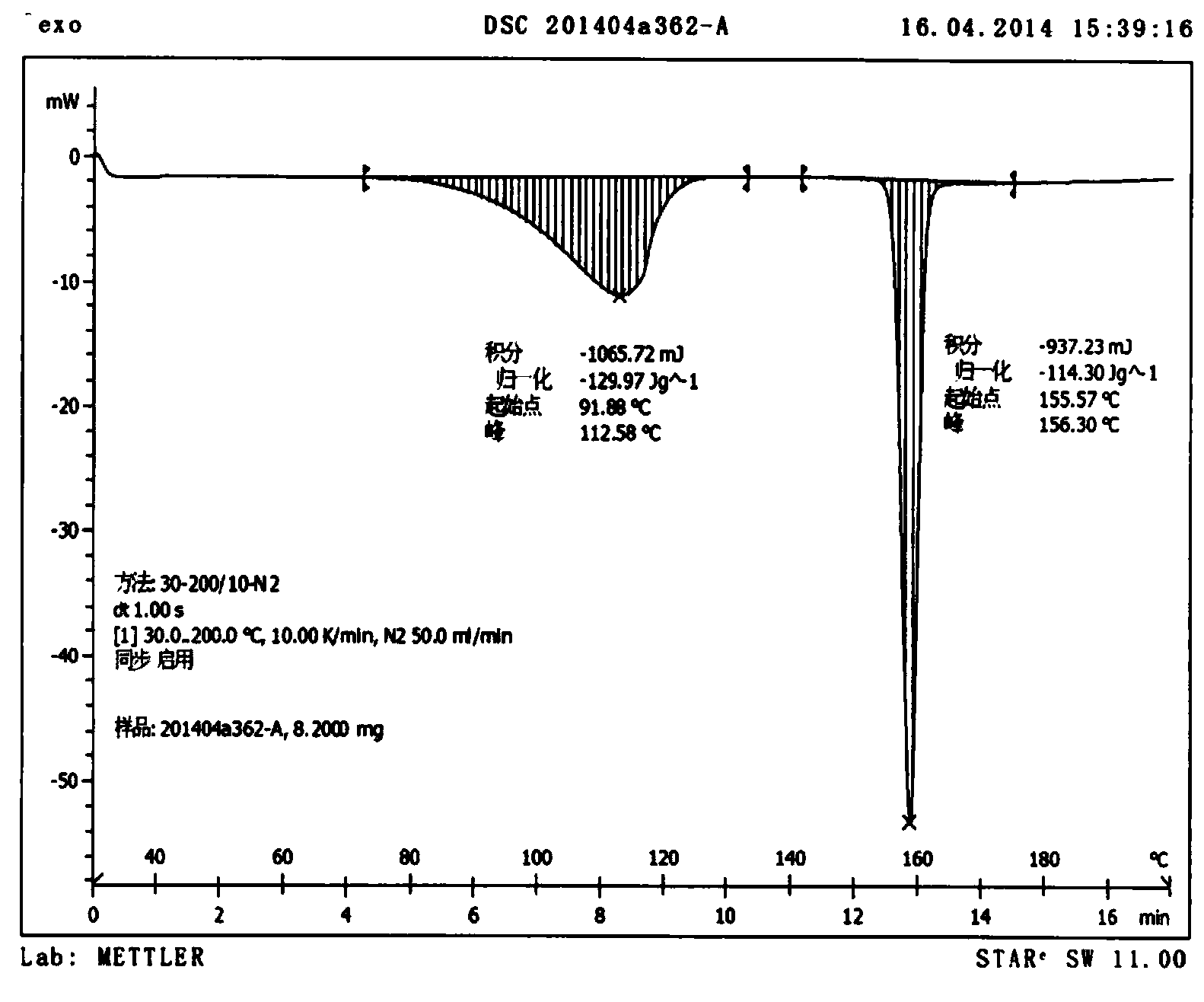
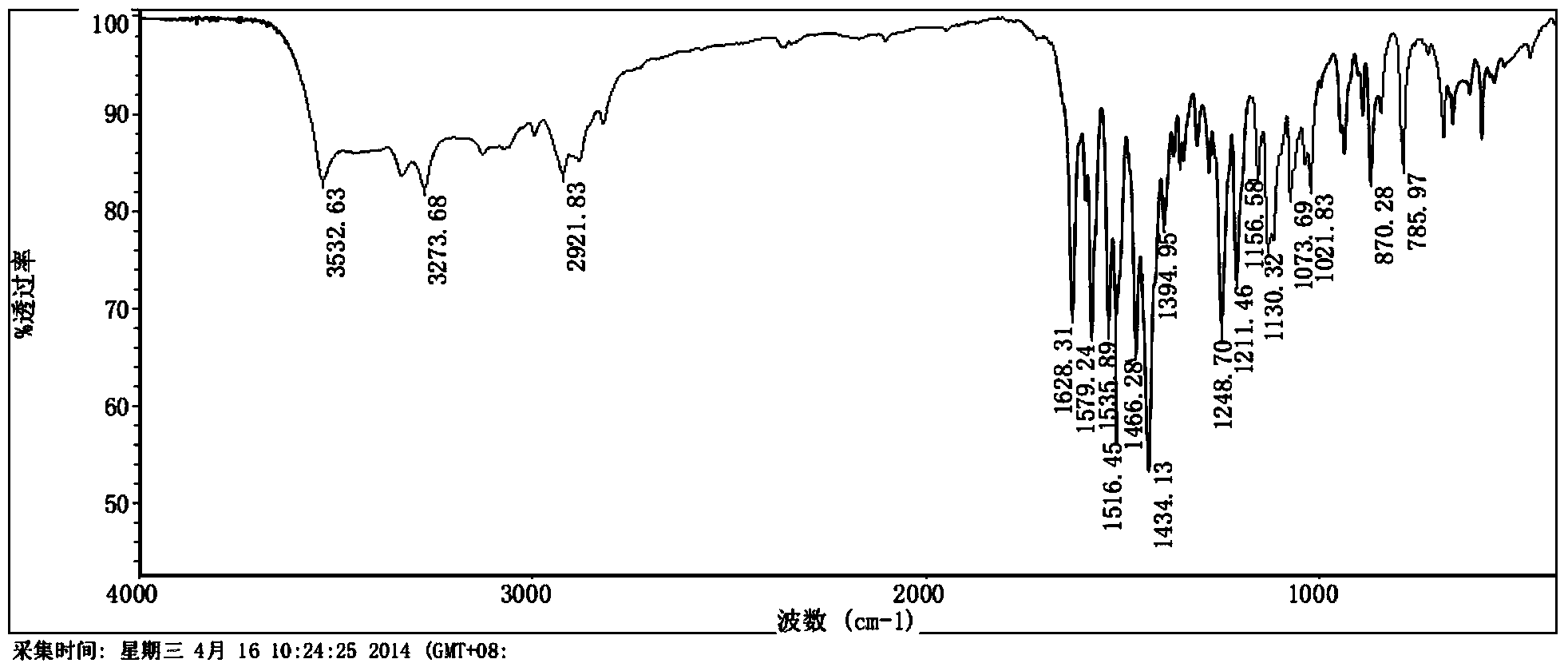
Preparation method of erlotinib base monohydrate crystal form I
A technology for erlotinib base and monohydrate, which is applied in the field of preparation of erlotinib base monohydrate crystal form Form I, can solve the problems of single solvent type, complicated operation, lack of extensiveness and the like, and achieves purity High, simple operation, safe and environmentally friendly process route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Add 10.0g of erlotinib hydrochloride, 0.93g of sodium hydroxide, and 60ml of methanol to a clean and dry 250ml reaction bottle, stir at room temperature for 0.5h, take 1.0ml of the reaction solution, add 0.5ml of water, and the reaction solution becomes clear to indicate the reaction After completion, the reaction solution was added dropwise to 120ml of water for 0.5h. After the dropwise addition was completed, under stirring, the temperature was lowered to 0-5°C, filtered with suction, and the filter cake was vacuum-dried at 20°C to obtain a white solid. 8.2 g, yield 89.5%, HPLC purity 99.8%.
Embodiment 2
[0032] Add 10.0g of erlotinib hydrochloride, 1.24g of sodium carbonate, 60ml of methanol to a clean and dry 250ml reaction bottle, stir at room temperature for 1.0h, take 1.0ml of the reaction solution, add 0.5ml of water, the reaction solution becomes clear, indicating that the reaction is complete , add the reaction solution dropwise to 240ml of water, and the dropping time is 0.5h. After the dropwise addition, under stirring, cool down to 0-5°C, filter with suction, and vacuum-dry the filter cake at 20°C to obtain a white solid, 8.3 g, yield 90.6%, HPLC purity 99.7%.
Embodiment 3
[0034] Add 10.0g of erlotinib hydrochloride, 1.4g of sodium hydroxide, and 30ml of dioxane to a clean and dry 250ml reaction bottle, stir at room temperature for 1.0h, take 1.0ml of the reaction solution, add 0.5ml of water, and the reaction solution becomes Clarify that the reaction is complete. Add the reaction solution dropwise to 60ml of water for 0.5h. After the dropwise addition, under stirring, cool down to 0-5°C, filter with suction, and dry the filter cake in vacuum at 50°C to obtain White solid, 8.1 g, yield 88.4%, HPLC purity 99.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com