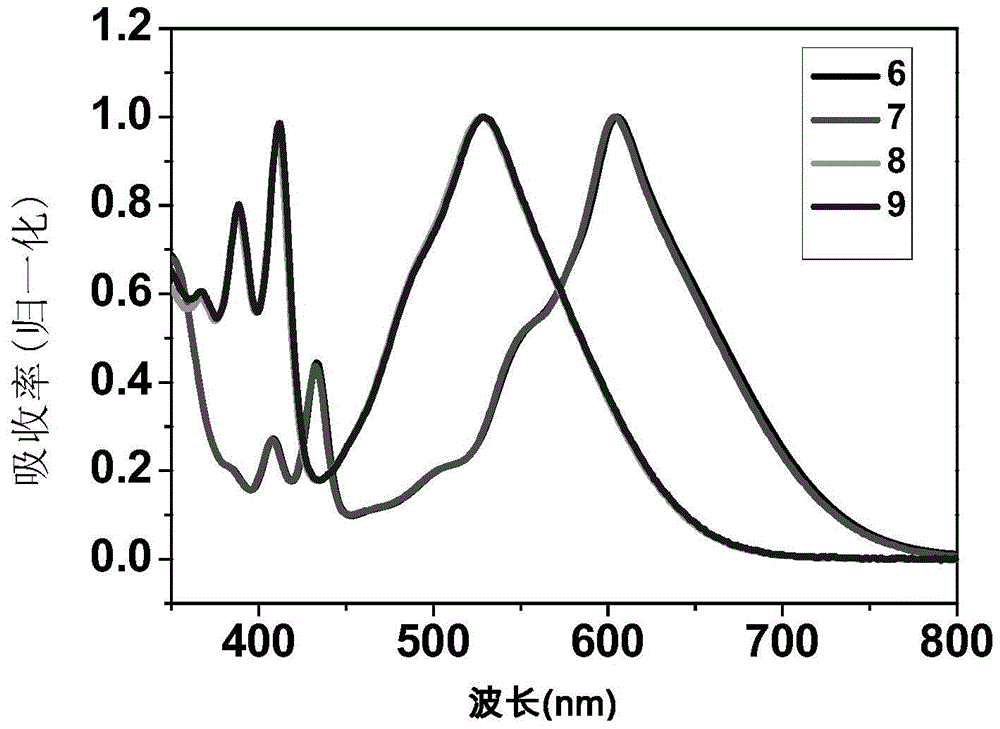
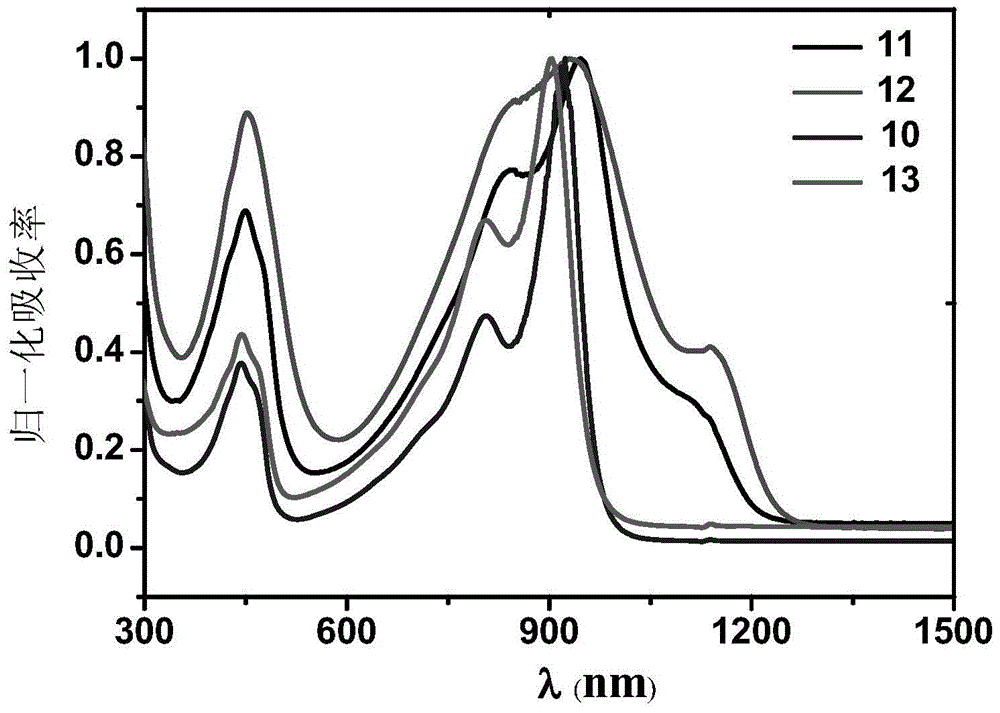
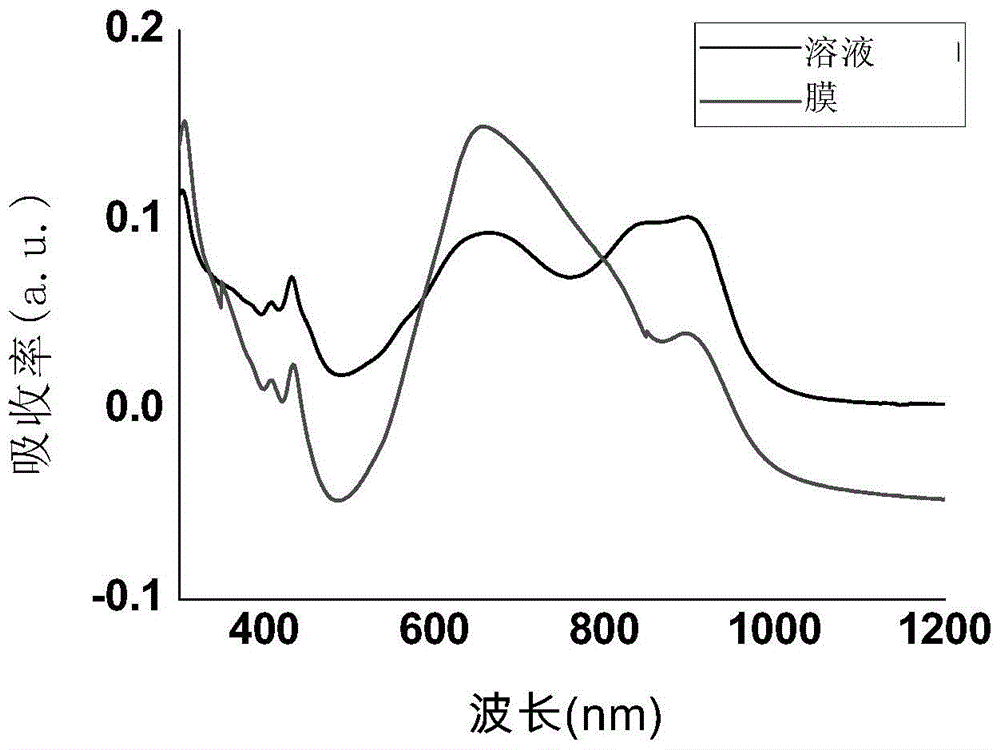
Naphthalene diimides and derivatives thereof containing 2-(1,3-disulfide/selenium-2-ylidene) acetocyanide conjugated structural units
A kind of naphthalene diimide, C2-C48 technology, applied in the field of organic electronic materials, can solve the problem of lacking, reducing the polymerization monomer and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0217] The preparation of formula (A) compound
[0218] The present invention also provides a preparation method of a compound of formula (A), comprising the steps of:
[0219]
[0220] (a) in an inert solvent, react with a compound of formula Ia and a halogenating reagent to obtain a compound of formula I or a compound of formula II;
[0221] or include steps:
[0222]
[0223] (b) in an inert solvent, react with a compound of formula (IIIa) and a halogenating reagent to obtain a compound of formula (IIIb);
[0224] Wherein, M is CN or H, and the definitions of other groups are as described in the first aspect of the present invention.
[0225] In another preferred example, the steps are performed in air or under the protection of an inert gas.
[0226] In another preferred embodiment, the inert solvent is a chlorine-containing organic solvent, preferably selected from the group consisting of chloroform and carbon tetrachloride.
[0227] In another preferred example...
Embodiment 1
[0330] Example 1: N-(2-decyl-dodecyl)-[2,3-d:6,7-d']-bis[2-(1,3-dithio-2-ylidene) Synthesis of -2-bromoacetocyanide]-naphthalene-1,4,5,8-tetracarboxylic diimide (1)
[0331]
[0332] 1mL Br at room temperature 2 (179mg, 1.12mmol) in chloroform solution was added dropwise to N-(2-decyl-dodecyl)-[2,3-d:6,7-d′]-bis[2-(1,3 -Dithio-2-ylidene) acetocyanide]-naphthalene-1,4,5,8-tetracarboxylic diimide (568mg, 0.487mmol) in 8mL chloroform solution, react for 2h, stop the reaction, reduce pressure The solvent was distilled off, and the remaining solid was subjected to column chromatography with petroleum ether / dichloromethane (1 / 1) as eluent to obtain 594 mg of product (blue solid) with a yield of 92%. 1 HNMR (300MHz, CDCl 3 )δ(ppm):0.84–0.87(t,12H),1.22(br,80H),2.02(br,2H),4.17(br,4H); 13 C NMR (100MHz, CDCl 3 ): δ: 14.10, 22.68, 26.34, 29.36, 29.63, 29.66, 29.67, 29.70, 29.71, 30.10, 31.44, 36.49, 31.53, 31.91, 36.40, 36.44, 36.49, 46.29, 71.24, 1114.14.14, 2 124.75, 124.98,...
Embodiment 2
[0333]Example 2: N-(2-octyl-dodecyl)-[2,3-d]-[2-(1,3-dithio-2-ylidene) propanedicyanide]-[6, 7-d'][2-(1,3-dithio-2-ylidene)-2-bromoacetonitrile]-naphthalene-1,4,5,8-tetracarboxylic diimide (2) Synthesis
[0334]
[0335] The compound N-(2-octyl-dodecyl)-[2,3-d]-[2-(1,3-dithio-2-ylidene) propanedicyanide]-[6,7- d'][2-(1,3-dithio-2-ylidene)acetocyanide]-naphthalene-1,4,5,8-tetracarboxylic diimide (30mg, 0.028mmol) was dissolved in 3mL chloroform 0.16 mL of a chloroform solution (V%=0.01) containing liquid bromine (0.031 mmol) was slowly added dropwise to the above solution, and the reaction solution was refluxed and stirred for 1 hour, then cooled to room temperature, and the solvent was removed under reduced pressure, and dichloromethane / Petroleum ether (1 / 1) was used as eluent, and the crude product was separated and purified by silica gel chromatography to obtain 28 mg of compound (2), with a yield of 88%. 1 H-NMR (300MHz, CDCl 3 )δ(ppm):0.86–0.88(m,6H,–CH 3 ),1.25–1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com