Gradient-doping positive material of lithium ion battery and preparation method of gradient-doping positive material of lithium ion battery
A technology for lithium ion batteries and cathode materials, which is applied in battery electrodes, secondary batteries, circuits, etc., can solve the problems of limited application scope, complex production process, and difficulty in mixing, etc., and achieves wide application scope, high discharge capacity, and cost. low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
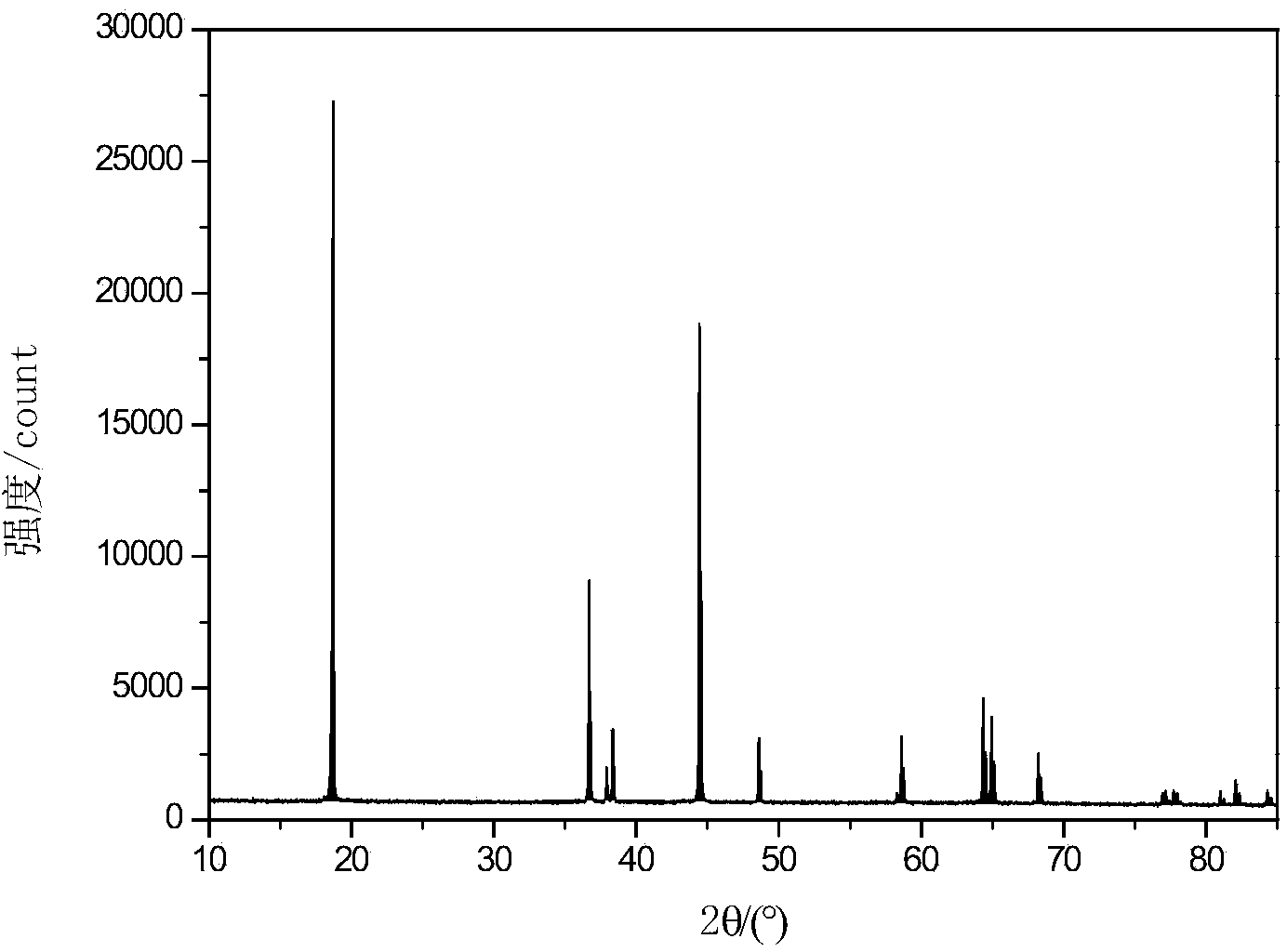
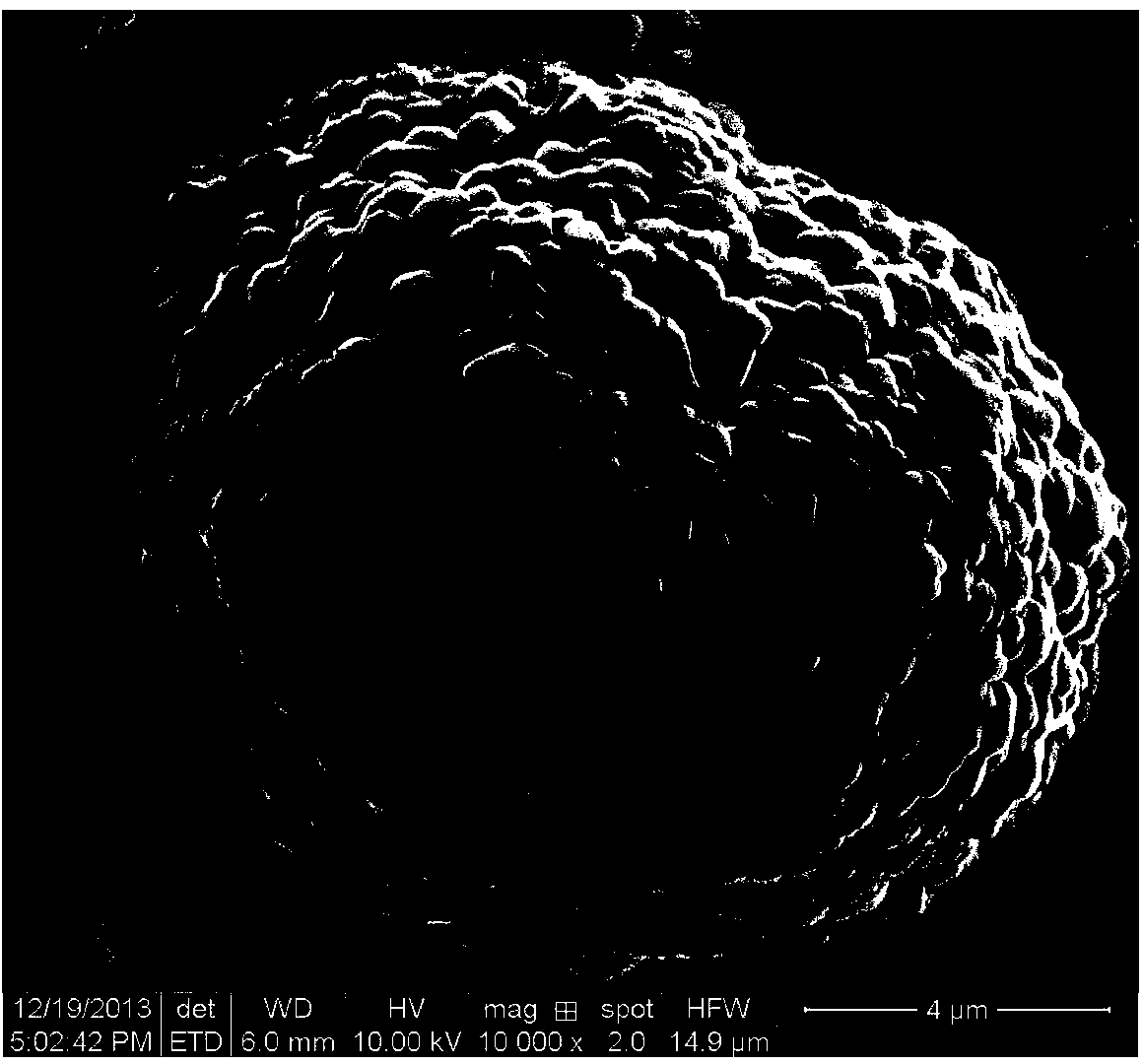
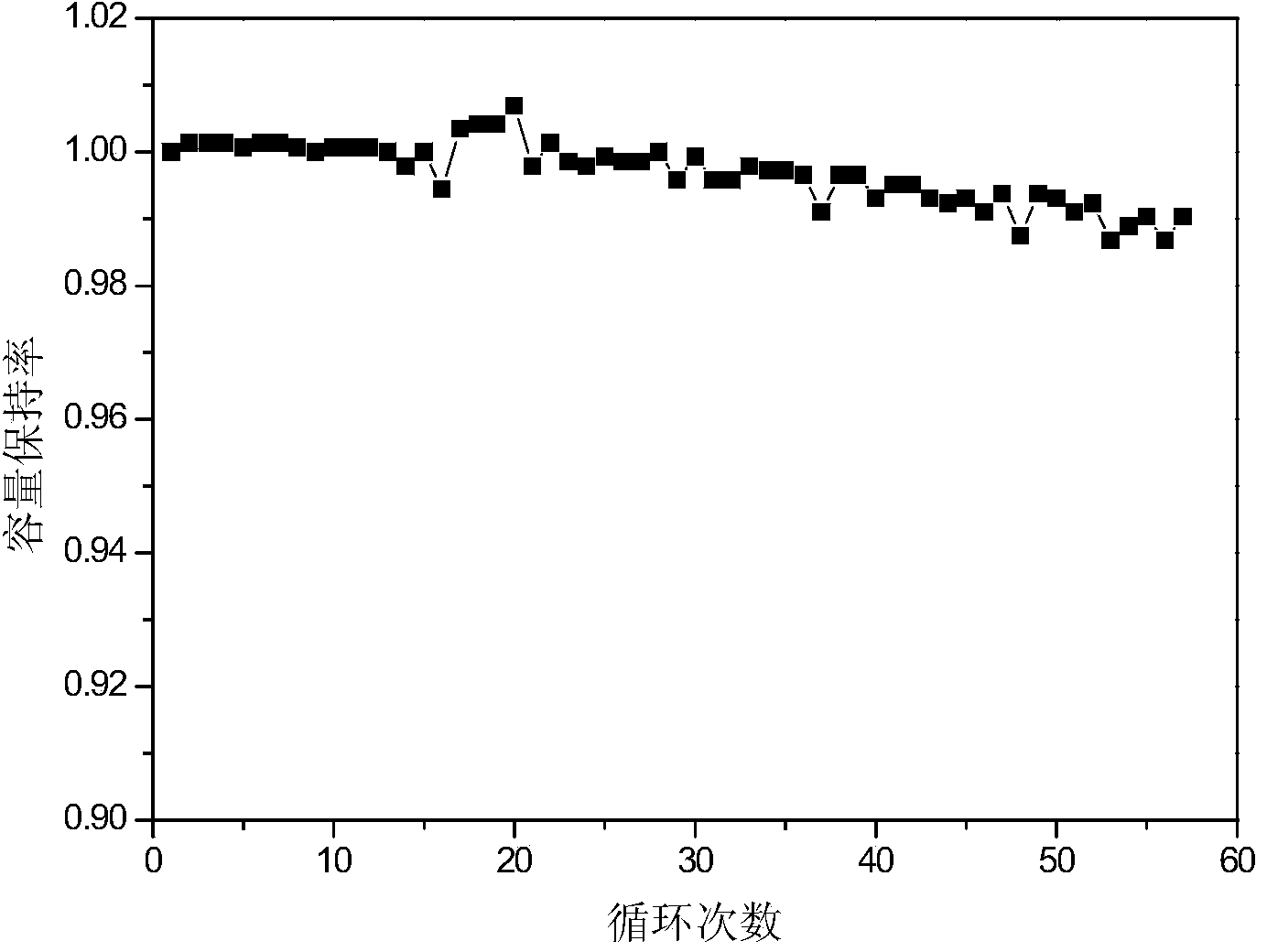
Image
Examples
Embodiment 1
[0037] (1) 92.8g precursor Ni 0.8 co 0.20 (OH) 2 Mix it with 35.2g of lithium carbonate by ball milling in a dry method. After the mixing is completed, place the above material in a sintering furnace and sinter at 700°C for 15 hours. The sintered material is crushed and passed through a 200-mesh sieve to obtain Li 0.95 Ni 0.80 co 0.20 o 2 .
[0038] (2) 81.7g C 4 h 6 MnO 4 .4H 2 O was added to deionized water and stirred to make a uniform solution, and the material to be doped in (1) was poured into the above solution, stirred for 2 hours, so that the material to be doped was fully impregnated in the manganese acetate solution, and then the mixed material was evaporated to dryness with stirring The moisture in the liquid forms the product Li covered with Mn element 0.95 Ni 0.80 co 0.20 o 2 .
[0039] (3) the above-mentioned Li covered with Mn element 0.95 Ni 0.80 co 0.20 o 2 Mix with 22.2g of lithium carbonate ball mill, after the mixing is completed, put the...
Embodiment 2
[0041] (1) 118.4g precursor Ni 0.80 co 0.10 mn 0.10 CO 3 Mix it with 37g of lithium carbonate by ball milling in a dry method. After the mixing is completed, put the above materials in a sintering furnace and sinter at 800°C for 12 hours. 0.80 co 0.10 mn 0.10 o 2 .
[0042] (2) Add 16.0g of aluminum isopropoxide to ethanol and stir to make a uniform solution, pour the material to be doped in (1) into the above solution, stir for 2 hours, and make the material to be doped in the aluminum isopropoxide solution Fully impregnated in the mixture, and then stirred and evaporated to dry the water in the mixture liquid to form the product LiNi coated with Al element 0.80 co 0.10 mn 0.10 o 2 .
[0043] (3) LiNi covered with Al element 0.80 co 0.10 mn 0.10 o 2 Mix it with 5.9g of lithium carbonate by ball milling in a dry method. After the mixing is completed, place the above material in a sintering furnace and sinter at 600°C for 5 hours. After the sintered material is c...
Embodiment 3
[0045] (1) Precursor 56.0gNiO, 21.7gMnO 2 Mix with 38.9g of lithium carbonate ball mill, after the mixing is completed, put the above material in a sintering furnace and sinter at 900°C for 10h, the sintered material is crushed and passed through a 200 mesh sieve to obtain the product to be doped LiNi 0.75 mn 0.25 o 2 .
[0046] (2) 72.8gCo(NO 3 ) 2 ·6H 2 O was added to deionized water and stirred to make a uniform solution, and the material to be doped in (1) was poured into the above solution, stirred for 2 hours, so that the material to be doped was fully impregnated in the cobalt nitrate solution, and then the mixed material was evaporated to dryness with stirring The moisture in the liquid forms the product LiNi coated with Co element 0.75 mn 0.25 o 2 .
[0047] (3) LiNi covered with Co element 0.75 mn 0.25 o 2 Mix it with 6.9g of lithium carbonate by ball milling in a dry method. After the mixing is completed, put the above material in a sintering furnace and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com