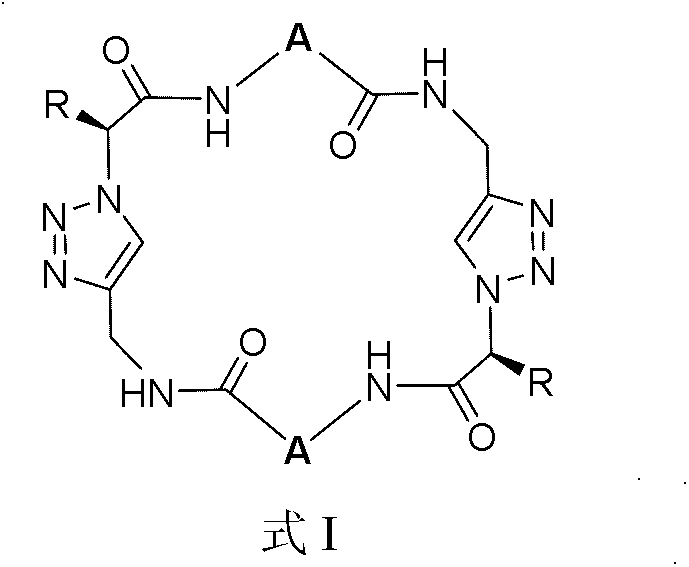
A flexible lactam macrocyclic molecule and its preparation method
A technology of lactams and macrocyclic molecules, which is applied in the direction of organic active ingredients, medical preparations containing active ingredients, and pharmaceutical formulas, and can solve problems such as complex steps, low reaction yields, and many side reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
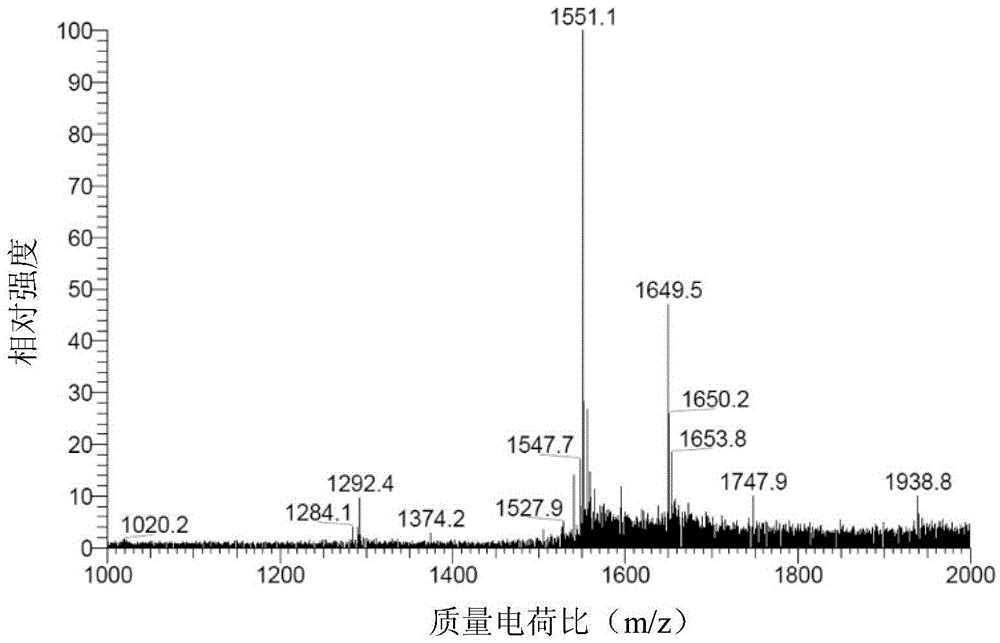
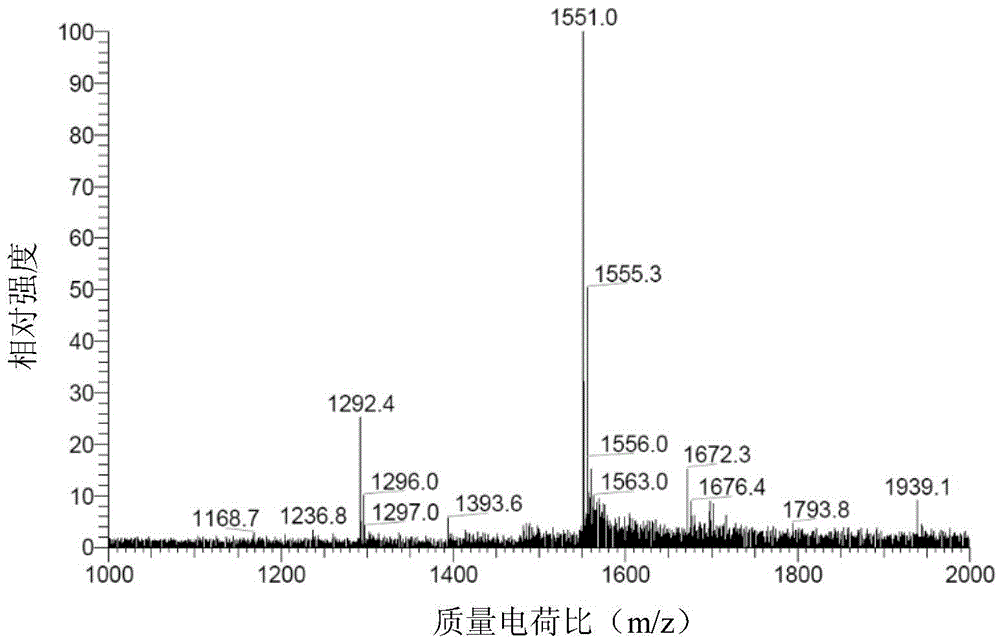
Image
Examples
preparation example Construction
[0043] Two, the preparation of raw material azidoacetic acid:
[0044] Reaction formula:
[0045]
[0046] Synthesis of Compound 10:
[0047] Dissolve 20.0 g of ethyl bromoacetate (120 mmol) in 20 mL of DMF in an ice-water bath, add sodium azide (12.5 g, 192 mmol) in batches, naturally rise to room temperature and react for 24 hours, then add saturated sodium carbonate solution, extract with ether three times, combine The organic phase was washed with saturated brine, dried over anhydrous sodium sulfate, and spin-dried to obtain a colorless transparent liquid, which was dissolved in 60mL of methanol, added with 60mL of 1M sodium hydroxide solution, reacted at 40°C for 5 hours, and the methanol was spin-off with Wash the aqueous phase twice with ether, neutralize the aqueous phase with 2M hydrochloric acid solution to a pH of about 2, extract three times with ether, combine the organic phases, wash with saturated brine, dry over anhydrous sodium sulfate, and spin dry to obt...
Embodiment 1
[0061] The synthesis of embodiment 1 compound 1 and 2
[0062] Reaction formula:
[0063]
[0064] 1. Synthesis of intermediate 14:
[0065] The basic building block molecular material 9 (1.00g, 4.42mmol), HOBt (0.600g, 4.44mmol) and HBTU (2.50g, 6.60mmol) were dissolved in DMF (7mL), and DIEA (0.7mL, 4mmol) was added to react for 15min Add propargylamine (0.42mL, 0.361g, 6.63mmol) to react overnight at room temperature under nitrogen protection, add ethyl acetate to the reaction system, wash with 1% hydrochloric acid solution, saturated sodium bicarbonate solution and saturated brine successively, anhydrous sulfuric acid Na-dried, spin-dried, and subjected to column chromatography (CH 2 Cl 2 :CH 3 OH=15:1-10:1) to obtain product 14 (1.12 g, 4.26 mmol, 96%) as a pale yellow solid. 1 HNMR (400MHz, DMSO-d 6 ): δ11.10(s, 1H), 9.01(s, 1H), 8.42(t, 3 J(H,H)=8.0Hz,1H), 6.81(s,1H), 6.70(s,1H), 3.96(s,2H), 3.09(s,1H), 1.44(s,9H). 13 CNMR (100MHz, DMSO-d 6 ): δ171.6, 163.7,...
Embodiment 2
[0074] The synthesis of embodiment two compounds 3 and 4
[0075] Reaction formula:
[0076]
[0077] 1. Synthesis of intermediate 17:
[0078] 2.0 g of Boc-protected propargylamine 13 (12.9 mmol) and 1.3 g of azidoacetic acid 10 (12.9 mmol) were dissolved in 10 mL of THF, and 2 mL of water, CuCl (0.64 g, 6.5 mmol), N, N'- Dimethylethylenediamine (1.4mL, 114g, 12.9mmol), reacted at room temperature for 1 hour, added 2mL of 2M hydrochloric acid solution, added ethyl acetate, washed with 1% hydrochloric acid and saturated brine successively, dried over anhydrous sodium sulfate and spun Drying and freezing gave the product 17 as a white solid (3.0 g, 11.7 mmol, 91%). 1 HNMR (400MHz, acetone-d 6 ): δ11.91(br, 1H), 7.96(s, 1H), 6.50(s, 1H), 5.33(s, 2H), 4.36(s, 2H), 1.44(s, 9H). 13 CNMR (100MHz, acetone-d 6 ): δ168.8, 156.8, 124.9, 82.0, 79.1, 51.2, 36.7, 28.2. LRMS (ESI-IonTrap): m / z255.0 ([M-H] - , C 10 h 15 N 4 o 4 The theoretically calculated value is 255.1).
[0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com