Carbon-clad spinel lithium titanate material, production method and application thereof
A lithium titanate and lithium titanate technology, which is applied to carbon-coated spinel lithium titanate materials and their production and application fields, can solve the problem that the material does not have a crystal form, and the rate characteristics cannot meet the requirements of high-power lithium-ion batteries, The impact of material cycle performance and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
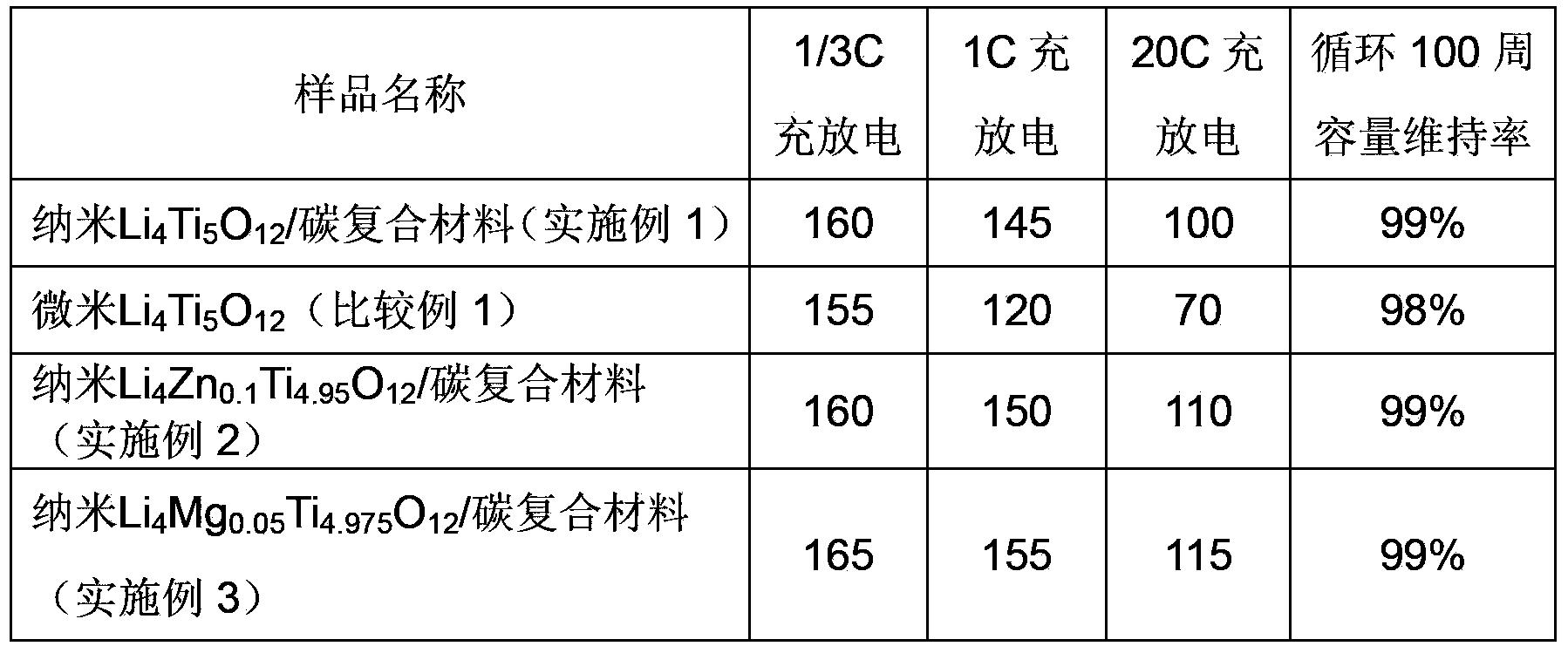
[0074] Adopt the method in the claim to synthesize nanometer Li 4 Ti 5 o 12 / carbon composite. The specific steps are as follows: add lithium acetate dihydrate (CH 3 COOLi·2H 2 O, 29.48g, 0.289mol), inject 200mL of absolute ethanol, and stir rapidly at 20°C; dropwise add tetrabutyl titanate ((C 4 h 9 O) 4 Ti, 120mL, 0.3526mol) (Li:Ti=0.82, molar ratio), added dropwise within five minutes. Add 24mL deionized water and 20mL glacial acetic acid to 160mL absolute ethanol, and stir evenly with a glass rod. The solution was transferred to the dropping funnel, and injected into the flask under rapid stirring (20s injection was completed).
[0075] 20 DEG C of constant temperature stirring 3h, form transparent sol, obtain white gel after aging 24h. The aged gel was vacuum-dried at 80°C for 12 hours to obtain a white slightly light yellow powder, which became a fine white powder after ball milling for 1 hour to obtain a precursor. The obtained precursor powder was pre-sinter...
Embodiment 2
[0084] Adopt the method in the claim to synthesize nanometer Li 4 Zn 0.1 Ti 4.95 o 12 / carbon composite particles. Add lithium acetate dihydrate (CH 3 COOLi·2H 2 O, 29.48g, 0.289mol) and zinc acetate dihydrate ((CH 3 COO) 2 Zn·2H 2 O, 2.195g, 0.01moL), inject 200mL of absolute ethanol, and stir rapidly at 20°C; add tetrabutyl titanate ((C 4 h 9 O) 4 Ti, 118.8mL, 0.03491mol) (Li:Zn:Ti=4.1:0.1:4.95), added dropwise within five minutes. Add 24mL deionized water and 20mL glacial acetic acid to 160mL absolute ethanol, and stir evenly with a glass rod.
[0085] Remaining steps are with embodiment 1.
[0086] The obtained product has a particle size of 400-500 nm. Assemble the buckle according to the method described in Example 1 and perform an electrochemical test. The test results show that the material has a capacity of 160mAh / g when charged and discharged at 1 / 3C; 150mAh / g when charged and discharged at 1C; and 110mAh / g when charged and discharged at 20C. Under th...
Embodiment 3
[0088] Adopt the method in the claim to synthesize nanometer Li 4 Mg 0.05 Ti 4.975 o 12 / carbon composite nanoparticles. Add lithium acetate dihydrate (CH 3 COOLi·2H 2O, 29.48g, 0.289mol) and magnesium acetate tetrahydrate (Mg(CH 3 COO) 2 4H 2 O, 1.072g, 0.005moL), inject 200mL of absolute ethanol, and stir rapidly at 20°C; add tetrabutyl titanate ((C 4 h 9 O) 4 Ti, 119.4mL, 0.3508mol) (Li:Zn:Ti=4.1:0.05:4.975), added dropwise within five minutes. Add 24mL deionized water and 20mL glacial acetic acid to 160mL absolute ethanol, and stir evenly with a glass rod. Remaining steps are with embodiment 1. get nano Li 4 Mg 0.05 Ti 4.975 o 12 / carbon composite nanoparticles.
[0089] Remaining steps are with embodiment 1.
[0090] Assemble the buckle according to the method described in Example 1 and perform an electrochemical test. The test results show that the material has a capacity of 165mAh / g when charged at 1 / 3C; 155mAh / g when charged at 1C; and 115mAh / g whe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com