Levamlodipine besylate tablets and preparation method thereof
A technology of L-benzenesulfonic acid and dipine tablets, which can be used in pill delivery, cardiovascular system diseases, drug combination, etc., can solve the problems of unstable drug quality, high-temperature damage, etc., and achieve less impurities, low production cost, and simple operation. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] A preparation method of levamlodipine besylate, the method consists of the following steps:
[0019] Mix starch and sodium carboxymethyl starch, add it to the binder dextrin, and granulate to obtain blank granules without active ingredients; dry the prepared granules at 60°C, when the moisture content of the granules reaches 1-3%. Collect the material and granulate with a fast granulator;
[0020] Dissolving levamlodipine besylate in absolute ethanol to make levamlodipine besylate ethanol solution, spraying it into dry granules and adding additional excipients (magnesium stearate), mixing, and drying at room temperature;
[0021] The content of levamlodipine besylate in the mixed granules was measured, and the mixed granules with qualified content were compressed into tablets to make levamlodipine besylate tablets.
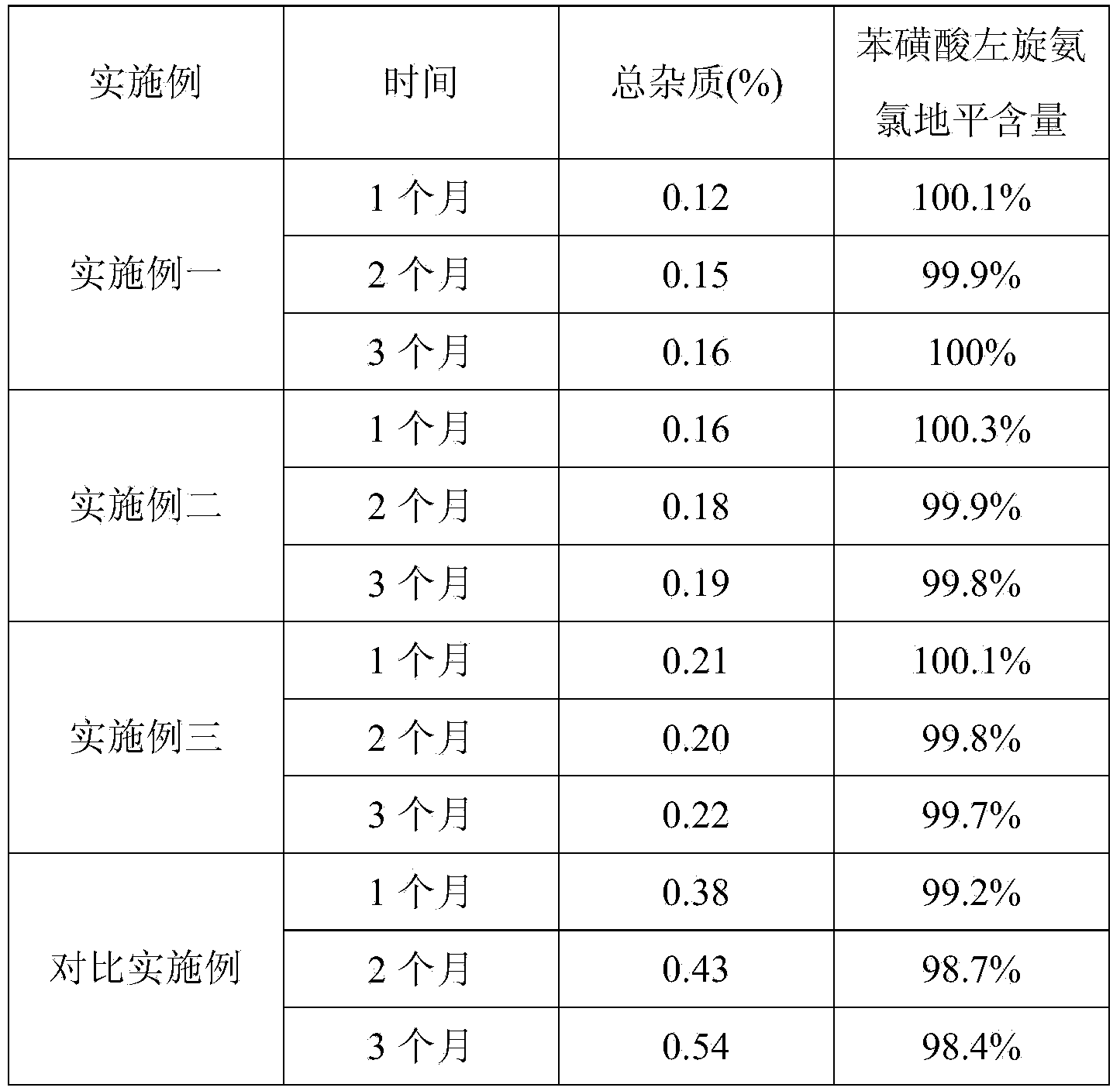
[0022] Get the prepared Levoamlodipine Besylate Tablets, and detect the total impurities of the Levoamlodipine Besylate Tablets. The sample had a total i...
Embodiment 2
[0027] Preparation of Levoamlodipine Besylate Tablets
[0028] Excipient granulation: mix lactose and croscarmellose sodium and add it to the binder starch to granulate; dry the granules at 70°C, collect the granules when the moisture content of the granules reaches 1-3%, and use fast Whole grain machine;
[0029] Dissolving levamlodipine besylate in absolute ethanol to make levamlodipine besylate ethanol solution, spraying it into dry granules and adding additional auxiliary materials (talc powder), mixing, and drying at normal temperature;
[0030] The content of levamlodipine besylate in the mixed granules was measured, and the mixed granules with qualified content were compressed into tablets to make levamlodipine besylate tablets.
[0031] Get the prepared levamlodipine besylate tablet sample, and detect the total impurities of the levamlodipine besylate tablet. The sample had a total impurity of 0.16%.
[0032] Judging from the detection results of the total impuritie...
Embodiment 3
[0037] A preparation method of levamlodipine besylate, the method consists of the following steps:
[0038] Excipient granulation: mix mannitol and crospovidone and add it to the binder hydroxypropyl cellulose to granulate; dry the prepared granules at 80°C, and collect when the moisture content of the granules reaches 1-3%. The material is granulated with a fast granulator;
[0039] Dissolving levamlodipine besylate in absolute ethanol to make levamlodipine besylate ethanol solution, spraying it into dry granules and adding additional auxiliary materials (silicon dioxide), mixing, and drying at room temperature;
[0040] The content of levamlodipine besylate in the mixed granules was measured, and the mixed granules with qualified content were compressed into tablets to make levamlodipine besylate tablets.
[0041] Get the prepared levamlodipine besylate tablet sample, and detect the total impurities of the levamlodipine besylate tablet. The sample had a total impurity of 0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com