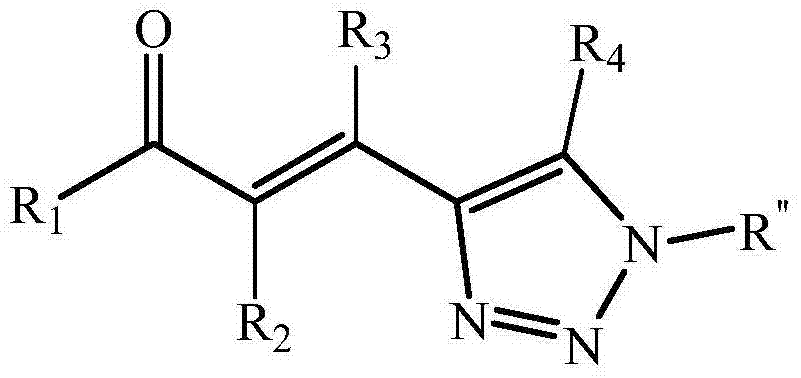
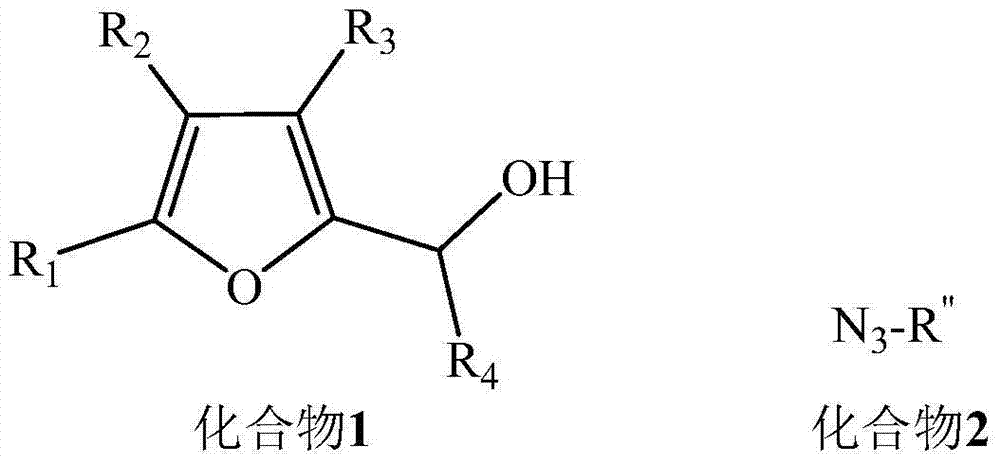
Ketene triazole compound and synthesis method thereof
A technology of enonetriazole and synthetic method, which is applied in the direction of organic chemistry, can solve the problems of complex starting materials, long synthetic routes, harsh reaction conditions, etc., and achieves a wide range of substrates, high step economy, and simple operation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
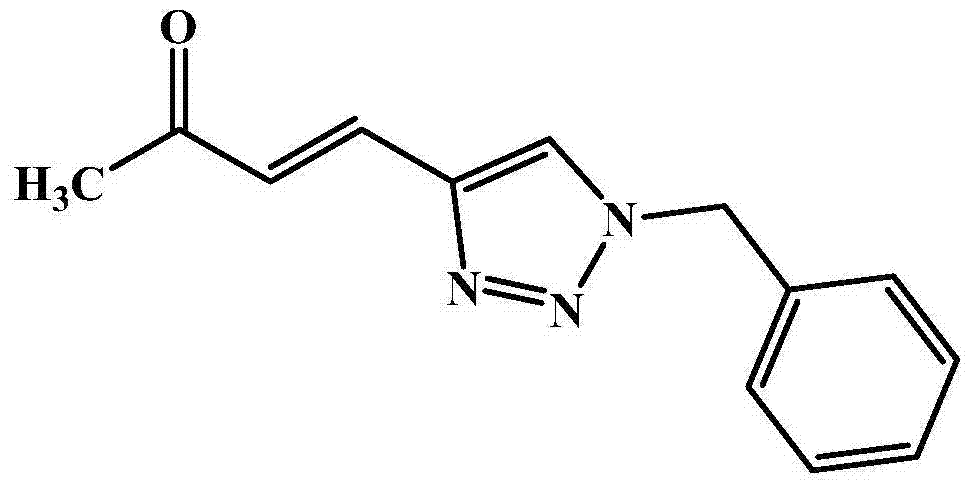
[0018] Taking (E)-4-(1-benzyl-1 hydrogen-1,2,3-triazol-4-yl)but-3-en-2-one as an example with the following synthetic structural formula, the raw materials used and their synthesis Methods as below:
[0019]
[0020] Add 112mg (1.0mmol) of 5-methyl-2-furylmethanol and 146mg (1.1mmol) of benzyl azide into 5mL of dichloromethane, place in a -20°C ice-salt bath, stir, and then dropwise add 1.1mL of 1mol / L TiCl 4 (1.1mmol) of dichloromethane solution, stirred at room temperature for 30 minutes after the dropwise addition, and the reaction mixture was washed with saturated NaHCO 3 Quenched, then diluted with 20mL ether, washed with saturated brine (2×5mL), extracted with ether (2×10mL), the organic phase was dried over anhydrous sodium sulfate and filtered, concentrated and washed with petroleum ether and ethyl acetate The mixed solution with a volume ratio of 1:1 was used as the eluent for column chromatography to obtain (E)-4-(1-benzyl-1hydrogen-1,2,3-triazol-4-yl)butan-3 ...
Embodiment 2
[0022] In Example 1, the TiCl used 4 with equimolar AlCl 3 Replacement, other steps are the same as in Example 1 to obtain (E)-4-(1-benzyl-1 hydrogen-1,2,3-triazol-4-yl)but-3-en-2-one, which The yield was 46%.
Embodiment 3
[0024] In Example 1, the TiCl used 4 with equimolar FeCl 3 Replacement, the reaction time was extended to 120 minutes, and other steps were the same as in Example 1 to obtain (E)-4-(1-benzyl-1 hydrogen-1,2,3-triazol-4-yl)butan-3- En-2-one in 51% yield.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com