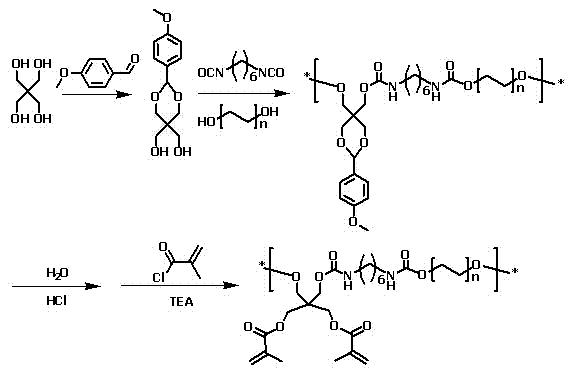
Rapid hydrophilic polyurethane light-curing adhesive, preparation method and curing method
A technology of adhesives and polyurethanes, applied in the direction of polyurea/polyurethane adhesives, adhesive types, adhesives, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
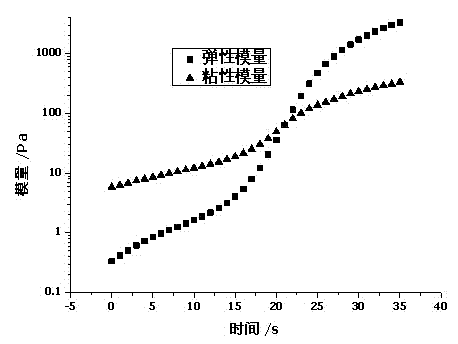
Image
Examples
Embodiment 1
[0032] Embodiment 1: the preparation of pentaerythritol acetal
[0033] Weigh 20.0 g of pentaerythritol powder, dissolve it in 400 mL of 0.1 mol / L hydrochloric acid solution, raise the temperature to 90° C., and stir for 1 hour to obtain a pentaerythritol hydrochloric acid solution with a mass concentration of 50 mg / mL. The pentaerythritol hydrochloric acid solution was cooled to 25°C, 20.0g anisaldehyde was added dropwise, and the stirring was continued for 2h. The obtained white powder was recorded as pentaerythritol acetal, and the precipitate was washed with 1 L of distilled water, and the mass of the white solid obtained after drying was 39.0 g, and the yield was 97.5%. The resulting white powder was placed in a desiccator for later use.
Embodiment 2
[0034] Embodiment 2: Preparation of binder precursor polyurethane
[0035] Weigh 6.0 g of the pentaerythritol acetal powder obtained in Example 1, 6.0 g of polyethylene glycol (molecular weight 1 kDa), and 4.4 g of HDI were dissolved in 50 mL of 1,2-dichloroethane and 50 mL of tetrahydrofuran to obtain a mass concentration of 164 mg / mL of the reaction system solution. 0.1 g of dibutyltin dilaurate was added dropwise as a catalyst, nitrogen gas was introduced into the reaction system, and the reaction was carried out at 80° C. for 24 hours. After the reaction, the reaction system solution was cooled to 25°C, and precipitated with 600 mL of anhydrous ether to obtain a white or light yellow waxy solid, which is the binder precursor polyurethane. After drying, 15.0 g of the solid mass was obtained, and the yield was 91.5%. . The obtained white or light yellow waxy solid was placed in a desiccator for later use.
Embodiment 3
[0036] Example 3: Hydrolysis of Adhesive Precursor Polyurethane
[0037] Weigh 8.0 g of the binder precursor polyurethane obtained in Example 2, dissolve it in 100 mL of hydrochloric acid solution with a concentration of 0.1 mol / L, configure it into a polyurethane hydrochloric acid solution with a mass concentration of 80 mg / mL, and stir it with 25 ° C After 12 hours, the polyurethane was hydrolyzed to release anisaldehyde. After the reaction, neutralize the reaction system to pH=7 with 10mL NaOH aqueous solution with a concentration of 1mol / L, remove the solvent water by rotary evaporation at 5kPa and 60°C, add 100mL tetrahydrofuran to dissolve the solid (the mass concentration of polyurethane is about 60mg / mL ), filtered to remove the NaCl precipitate insoluble in tetrahydrofuran, and then precipitated with 500mL of anhydrous ether to obtain a white or light yellow waxy solid, which is hydrolyzed polyurethane. After drying, 6.0 g of solid mass was obtained, and the yield wa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com