Medicinal application of A type proanthocyanidin trimer polyphenol
A procyanidin and application technology, applied in the direction of drug combination, medical preparations containing active ingredients, antipyretics, etc., can solve the problem of less polyphenols in oligomeric procyanidins and no A-type trimeric procyanidins Issues such as the report on the immunosuppressive effect of polyphenols, to achieve the effect of inhibiting arthritis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
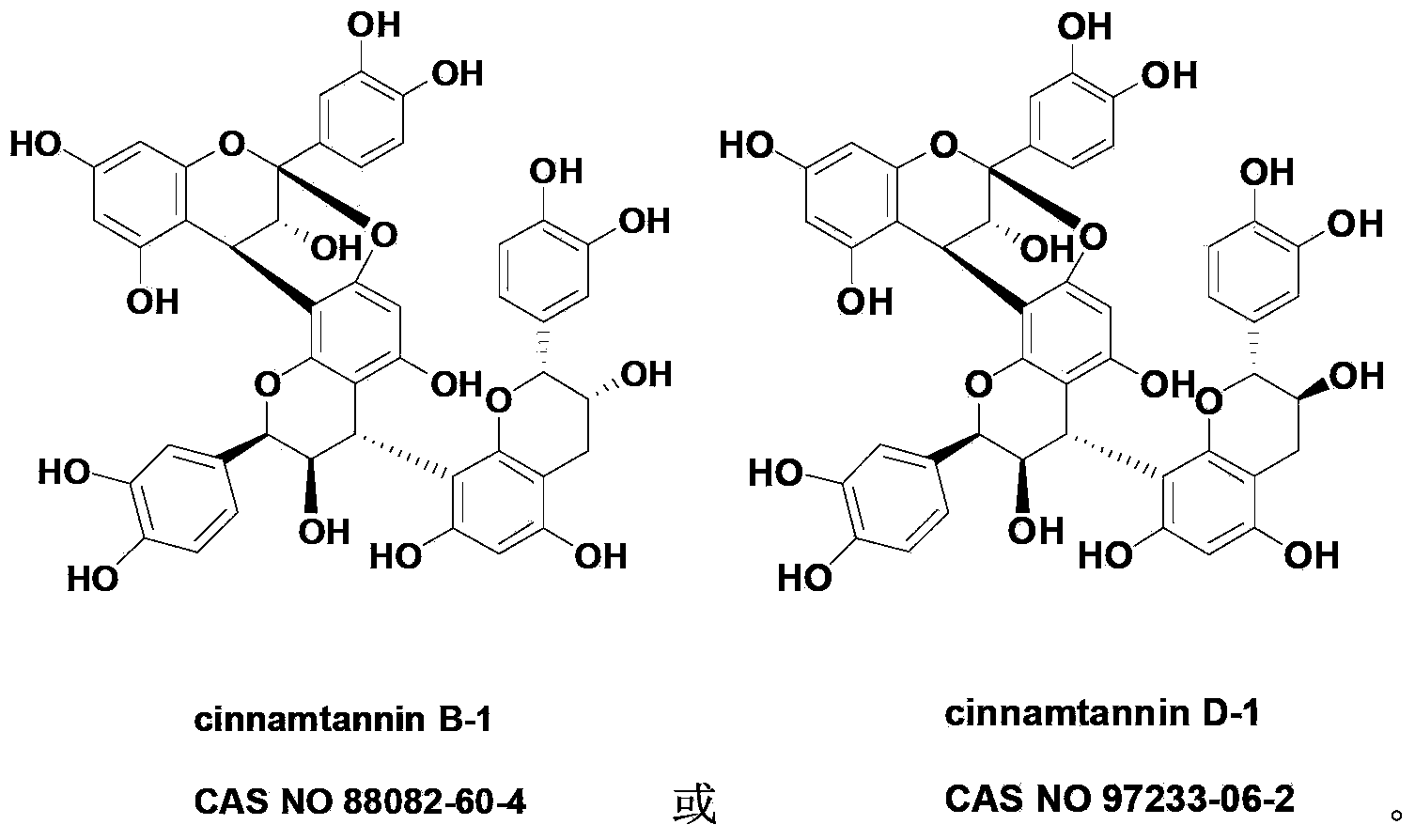
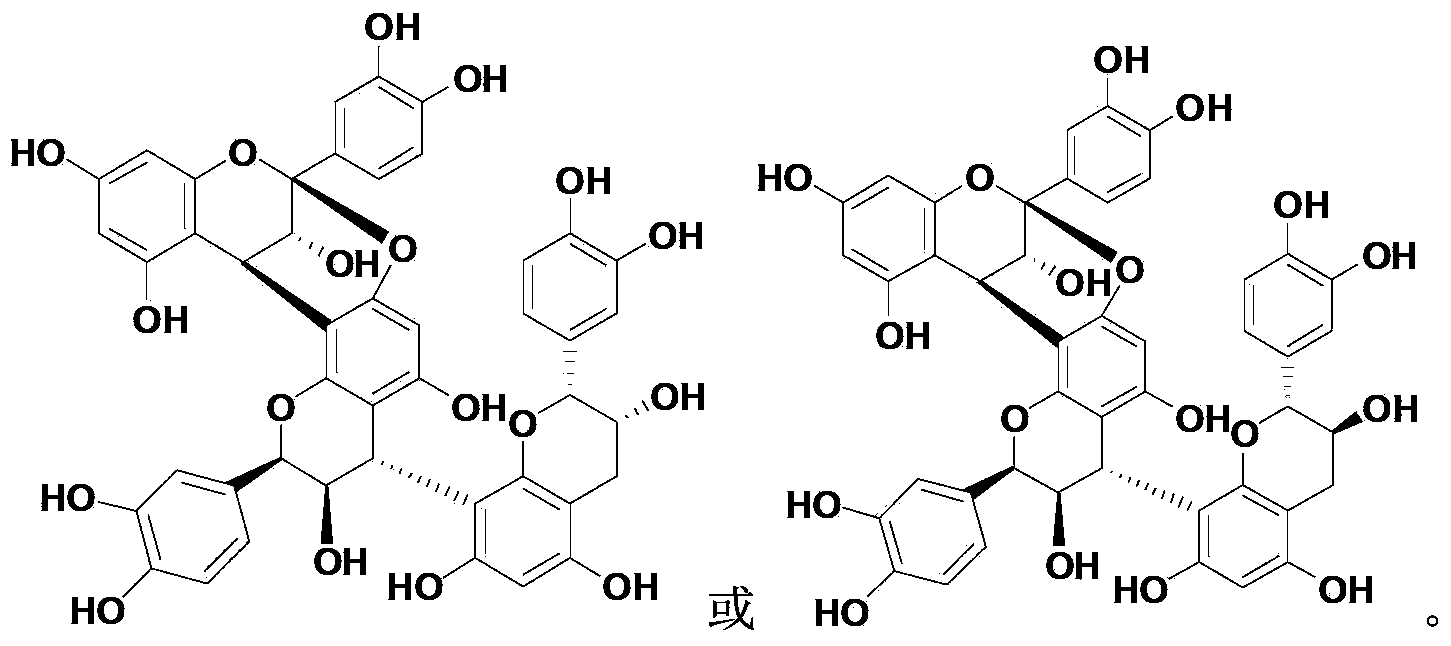
[0016] Example 1: Preparation of Compound Cinnamtannin B-1 (CAS No.: 88082-60-4)
[0017] 1.0 kg of Chaigui medicinal material was reflux extracted with 5L of 95vol% ethanol aqueous solution, refluxed for 2 hours each time, refluxed and extracted 3 times in total, the extracts were combined, concentrated under reduced pressure to obtain an extract (about 800 mL); After adding 1 times the amount of water to suspend, sequentially extract with petroleum ether (1000mL×3) and ethyl acetate (1000mL×3), collect the ethyl acetate extract; recover ethyl acetate under reduced pressure and perform silica gel column chromatography, Gradient elution was carried out with petroleum ether and ethyl acetate in sequence, received in sections, and combined to obtain 17 fractions; each fraction was separated by silica gel column chromatography and reverse phase silica gel column chromatography, and finally purified by Sephadex LH-20 , the compound Cinnamtannin B-1 was obtained as a white powder. ...
Embodiment 2
[0025] Example 2: Preparation of Compound Cinnamtannin D-1 (CAS No.97233-60-2)
[0026] Use 5L of 95vol% ethanol aqueous solution to reflux extract 10.0kg of Chaigui medicinal material, reflux for 2 hours each time, reflux extract for 3 times in total, combine the extracts, concentrate under reduced pressure to obtain extract (about 800mL); After adding 1 times the amount of water to suspend, sequentially extract with petroleum ether (1000mL×3) and ethyl acetate (1000mL×3), collect the ethyl acetate extract; recover ethyl acetate under reduced pressure and perform silica gel column chromatography, Gradient elution was carried out with petroleum ether and ethyl acetate in sequence, received in sections, and combined to obtain 17 fractions; each fraction was separated by silica gel column chromatography and reverse phase silica gel column chromatography, and finally purified by Sephadex LH-20 , the compound Cinnamtannin D-1 was obtained as a white powder.
[0027] After analysi...
Embodiment 3
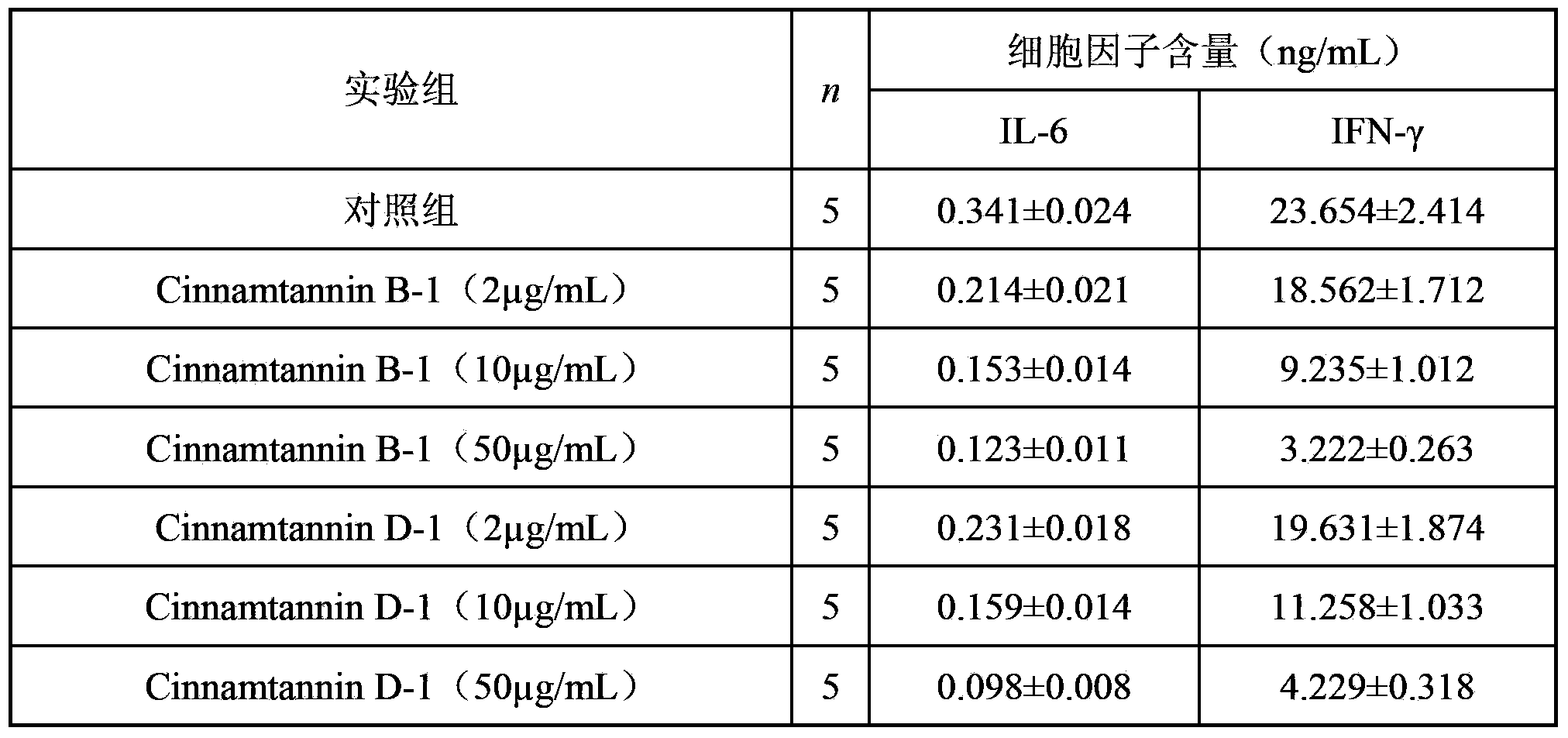
[0035] Detect the cytotoxicity of compound of the present invention with MTT method:
[0036] Sample group: DMSO solvent was used to prepare the compounds prepared in Examples 1 and 2 into solutions with concentrations of 12.5 μg / mL, 25 μg / mL and 50 μg / mL, respectively;
[0037] Control group: RPMI-1640 culture medium;
[0038] BALB / c mouse spleen lymphocytes (4 × 10 5 pc / well) were co-cultured with the samples of each experimental group for 48 h, and MTT solution (5 mg / mL) was added 4 h before the end of the culture. After the culture, 150 μL of the culture supernatant was aspirated, and 150 μL of DMSO was added to dissolve the formazan particles, shaken at a low speed for 10 min, and the OD value was read at 570 nm with a microplate reader.
[0039] The cell viability value (OD value) of each experimental group was detected, and the detailed detection results are shown in Table 1.
[0040] Table 1 The measured cell OD value of each experimental group after 48h of action ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com