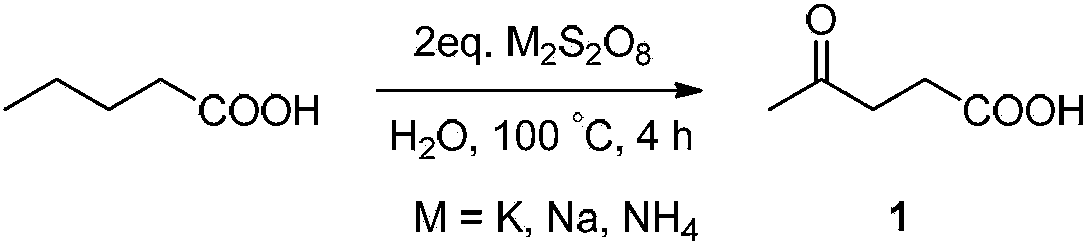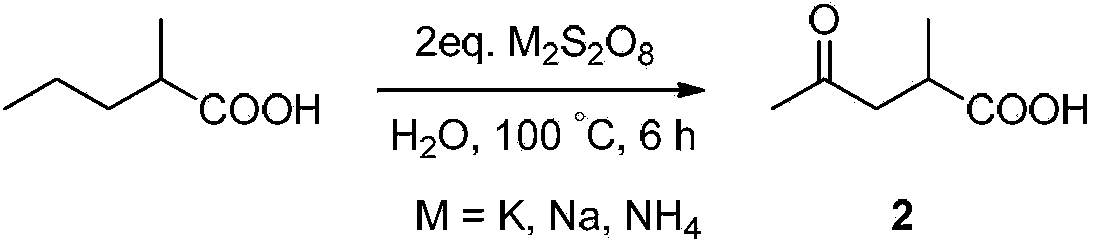Method for preparing gamma-carbonyl carboxylic acid, amino acid, amino acid ester and amide compounds
A carbonyl amino acid ester and carbonyl amino acid technology, which is applied in the field of preparation of γ-carbonyl carboxylic acid, amino acid ester and amide compounds, and amino acids, and can solve the problems of high price, limited application, difficult recovery and recycling of homogeneous catalysts, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment 1, the preparation of compound 1 (method one)
[0033] The reaction equation is as follows:
[0034]
[0035] Place 1 mmol of n-valeric acid (102 mg) in a 25 mL reaction vessel with a polytetrafluoroethylene stopcock, add 2 times the amount of oxidant potassium persulfate K 2 S 2 o 8 (2mmol, 540mg), dissolved in 5mL of distilled water and heated at 100°C for 4 hours. After the reaction, the reaction system was cooled to room temperature, extracted three times by adding ethyl acetate (10 mL×3 times), and the organic phase was washed with anhydrous Na 2 SO 4 After drying, it was spin-dried and purified by column chromatography (the volume ratio of dichloromethane:methanol was 15:1). The reaction was monitored by thin-layer chromatography, visualized with bromophenol blue or CAM.
[0036] The structural confirmation data of this product are as follows:
[0037] 1 H NMR (400MHz, CDCl 3 ): δ(ppm) 2.20(s, 3H), 2.26(t, J=6.4Hz, 2H), 2.76(t, J=6.4Hz, 2H)....
Embodiment 2
[0039] Embodiment 2, the preparation of compound 2 (method one)
[0040] The reaction equation is as follows:
[0041]
[0042] Put 1mmol of 2-methyl-n-valeric acid (116mg) in a 25mL reaction vessel with a polytetrafluoroethylene stopcock, add 2 times the amount of oxidant potassium persulfate K 2 S 2 o 8 (2mmol, 540mg), dissolved in 10mL of distilled water, and heated at 100°C for 6 hours. After the reaction, the reaction system was cooled to room temperature, extracted three times by adding ethyl acetate (10 mL×3 times), and the organic phase was washed with anhydrous Na 2 SO 4 After drying, it was spin-dried and purified by column chromatography (the volume ratio of dichloromethane:methanol was 15:1). The reaction was monitored by thin-layer chromatography, visualized with bromophenol blue or CAM.
[0043] The structural confirmation data of this product are as follows:
[0044] 1 H NMR (400MHz, CDCl 3 ): δ(ppm) 1.23(d, J=6.8Hz, 3H), 2.18(s, 3H), 2.44-2.55(m, 1H...
Embodiment 3
[0046] Embodiment 3, the preparation of compound 3 (method one)
[0047] The reaction equation is as follows:
[0048]
[0049] Put 1 mmol of 3-methyl-n-valeric acid (116 mg) in a 25 mL reaction vessel with a polytetrafluoroethylene stopcock, add 2 times the amount of oxidant potassium persulfate K 2 S 2 o 8 (2mmol, 540mg), dissolved in 10mL of distilled water, and heated at 100°C for 12 hours. After the reaction, the reaction system was cooled to room temperature, extracted three times by adding ethyl acetate (10 mL×3 times), and the organic phase was washed with anhydrous Na 2 SO 4After drying, it was spin-dried and purified by column chromatography (the volume ratio of dichloromethane:methanol was 15:1). The reaction was monitored by thin-layer chromatography, visualized with bromophenol blue or CAM.
[0050] The structural confirmation data of this product are as follows:
[0051] 1 H NMR (400MHz, CDCl 3 ): δ (ppm) 1.18 (d, J = 7.2Hz, 3H), 2.23 (s, 3H), 2.31-2....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com