Method for preparing toluene diisocynate from urea under catalysis of ionic liquid
A technology of toluene diisocyanate and ionic liquid, which is applied in the field of organic synthesis, can solve the problems of high cost, low yield, and environmental pollution, and achieve the effects of reducing production cost, good catalytic performance, and beneficial to industrial application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] The method for preparing toluene diisocyanate using ionic liquid catalysis described in this embodiment comprises the following steps:
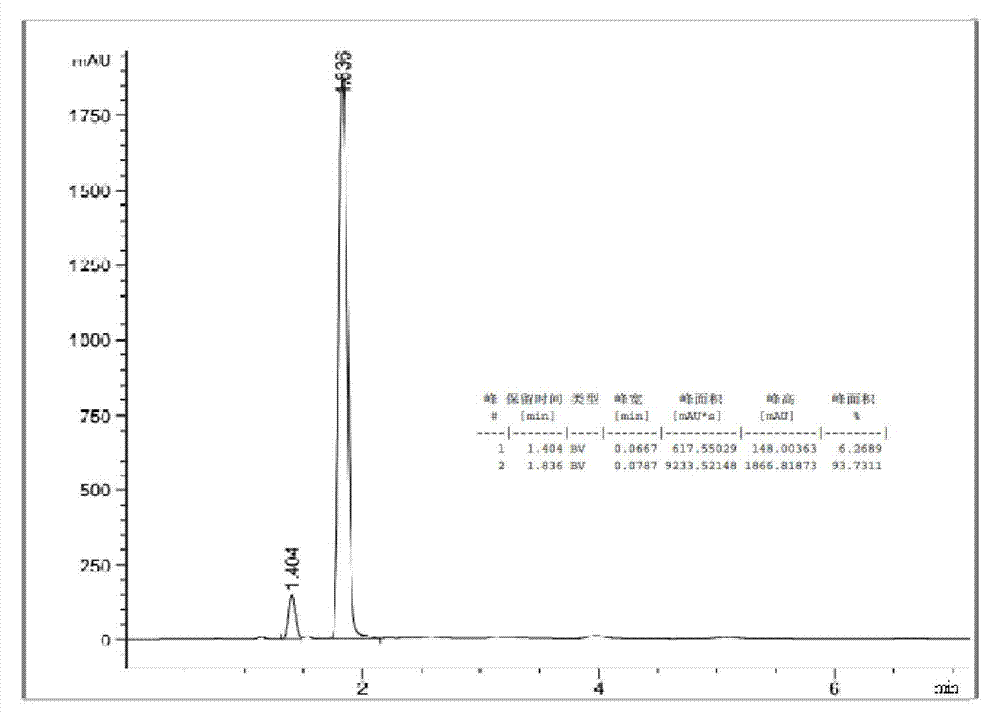
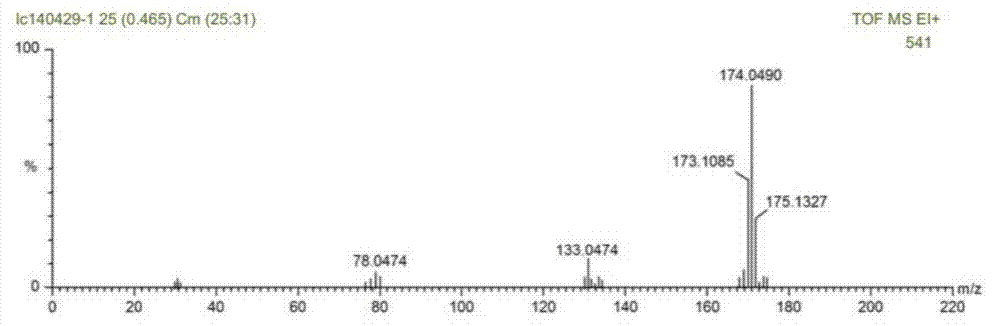
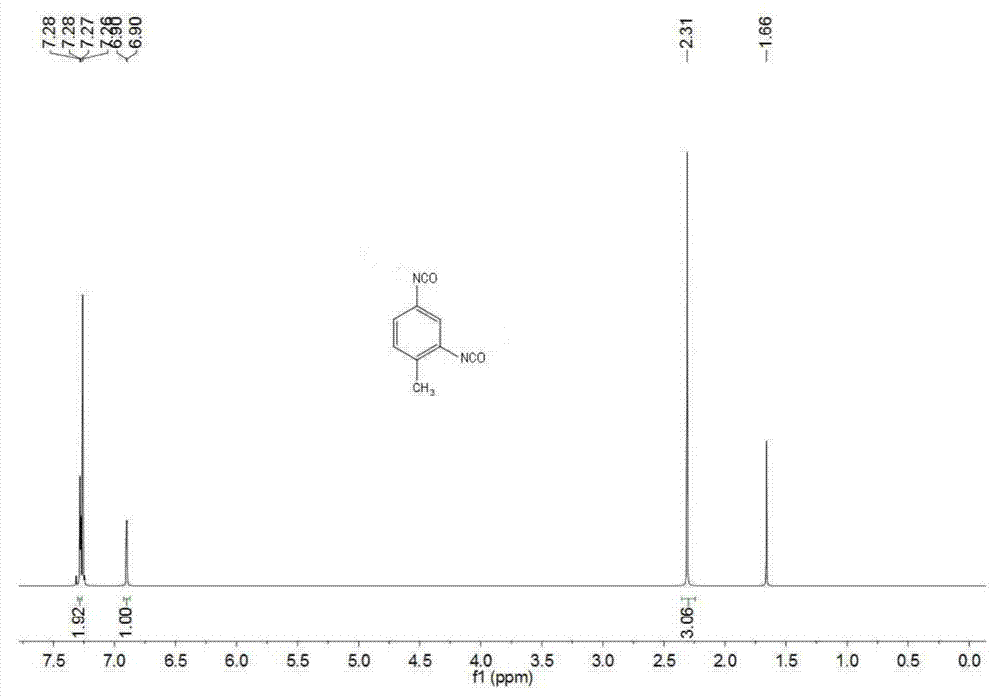
[0027] (1) Under the protection of nitrogen, add 244.3g of 2,4-toluenediamine, 300.3g of urea and 0.43g of 1-hexyl-2,3-dimethylimidazolium chloride successively to a 1L autoclave, and heat up Melt the solid in the reactor to a liquid state at 150°C, keep the pressure at 1 MPa, start stirring and count. Follow up the reaction process by liquid chromatography, stop stirring after reacting for 3 hours, treat the reaction solution to be cooled to room temperature, discharge the residual ammonia in the reactor, the above reaction solution is carried out to liquid chromatography analysis, the result is as follows figure 1 As shown, the conversion rate of toluenediamine in the reaction solution of step (1) is 96.8%, and the yield of tolenediurea is 93.7%. Among them, the ionic liquid 1-hexyl-2,3-dimethylimidazolium chloride salt was purchase...
Embodiment 2
[0030] The method for preparing toluene diisocyanate using ionic liquid catalysis described in this embodiment comprises the following steps:
[0031] (1) Under nitrogen protection, 244.3g of 2,6-toluenediamine, 360.4g of urea and 1.9g of 1-ethyl-3-methylimidazolium bromide were added successively to a 1L autoclave, and the temperature was raised to 140 °C to completely melt the solid in the reactor into a liquid state, keep the pressure at 1.5 MPa, start stirring and count the time. Track the reaction process by liquid chromatography, stop stirring after reacting for 10 hours, treat the reaction solution to be cooled to room temperature, discharge the residual ammonia in the reactor, liquid chromatography analysis obtains the conversion ratio of toluene diamine in the step (1) reaction solution to be 90.8 %, the yield of toluene diurea is 83.1%. Among them, the ionic liquid 1-ethyl-3-methylimidazolium bromide was purchased from Sigma-Aldirch, CAS: 143314-16-3.
[0032] (2) ...
Embodiment 3
[0034] The method for preparing toluene diisocyanate using ionic liquid catalysis described in this embodiment comprises the following steps:
[0035](1) Under nitrogen protection, 195.4 g of 2,4-toluenediamine, 48.9 g of a mixture of 2,6-toluenediamine, 336.4 g of urea and 1-butyl-3- Methylimidazolium hexafluorophosphate 1.4g, heated up to 130°C to completely melt the solid in the reactor into a liquid state, keep the pressure at 2MPa, start stirring and timing. Track the reaction process by liquid chromatography, stop stirring after reacting for 6.5 hours, treat the reaction solution to be cooled to room temperature, discharge the residual ammonia in the reactor, liquid chromatography analysis obtains the conversion rate of toluene diamine in the step (1) reaction solution 96.2 %, the yield of toluene diurea is 94.8%. Among them, the ionic liquid 1-butyl-3-methylimidazolium hexafluorophosphate was purchased from Sigma-Aldrich, CAS: 174501-64-5.
[0036] (2) Add the reactio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com