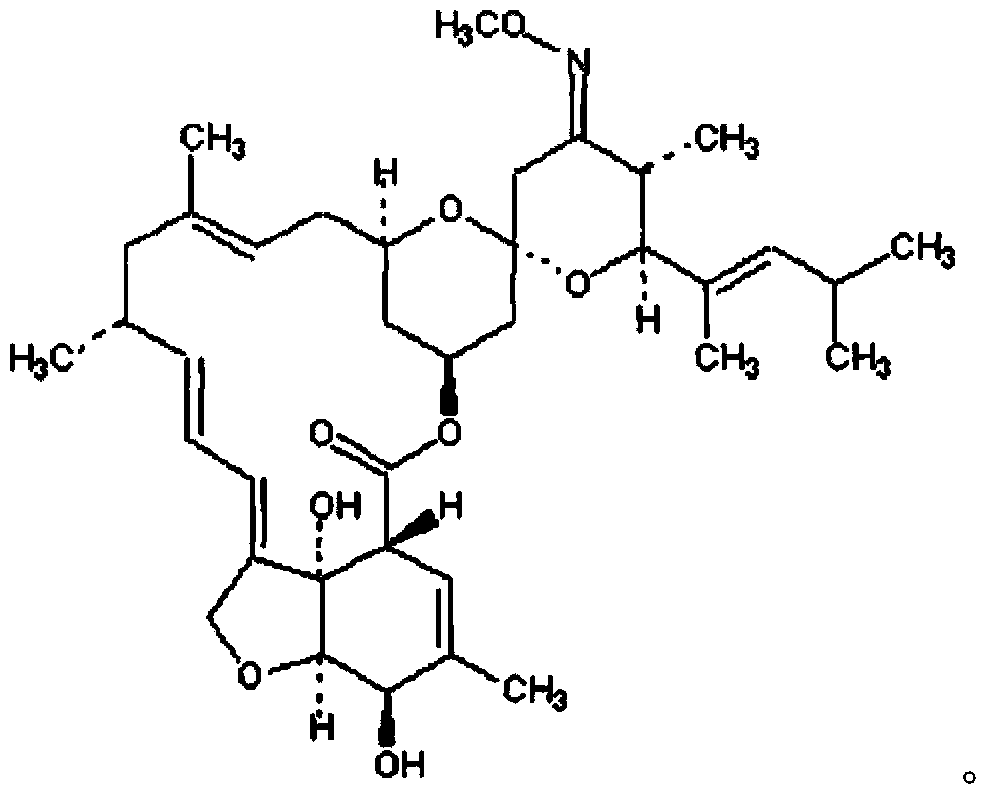
Method for preparation of moxidectin
A reaction and solution technology, applied in organic chemistry and other directions, can solve the problems of large amount of polar organic solvent, high solvent recovery pressure, complicated operation steps, etc., and achieve the effect of reducing production cost, improving purity and increasing product content.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0099] In a 1L three-necked reaction flask, put the solid content of Nimoctine (the weight percentage of Nimoctine is 80%, and the molar number is 0.098mol), 0.10mol of imidazole, and 300ml of dichloromethane and stir until dissolved at room temperature , and then dropwise added a solution of dichloromethane (400ml) containing tert-butyldimethylchlorosilane (0.26mol) while maintaining the temperature at 25°C, and stirred at room temperature for 9 hours after the dropwise addition was completed. TLC (thin plate chromatography) detection showed that the reaction of the raw materials was basically complete, and the resulting solution was washed with 1000 ml of purified water, and then dried and dehydrated with anhydrous sodium sulfate.
[0100] Add dimethylsulfoxide (0.11mol) and triethylamine (0.15mol) successively to the dehydrated solution, mix well and cool down to -30°C, add phenoxyphosphoryl dichloride (0.11mol) dropwise dichloromethane (39.56ml) solution, the reaction temp...
Embodiment 2
[0106] In a 1L three-necked reaction flask, put the solid matter of Nimoctine (the weight percentage of Nimoctine in it is 82.5%, and the molar number is 0.14mol), imidazole 0.14mol, and dichloromethane 425ml, and stir at room temperature until Dissolve, then add dropwise a solution of dichloromethane (550ml) containing tert-butyldimethylchlorosilane (0.37mol) while maintaining the temperature at 15°C, and stir at room temperature for 12 hours after the dropwise addition is completed. TLC (thin plate chromatography) detection showed that the reaction of the raw materials was basically complete, and the resulting solution was washed with 1420 ml of purified water, and then dried and dehydrated with anhydrous sodium sulfate.
[0107] Add dimethyl sulfoxide (0.15mol) and triethylamine (0.14mol) successively to the dehydrated solution, mix well and cool down to -30°C, add phenoxyphosphoryl dichloride (0.14mol) dropwise dichloromethane (52ml) solution, control the reaction temperat...
Embodiment 3
[0113] In a 1L three-necked reaction flask, put the solid matter of Nimoctine (the weight percentage of Nimoctine in it is 86%, and the molar number is 0.086mol), imidazole 0.091mol, and dichloromethane 265ml, and stir at room temperature until Dissolve, and then add dropwise a solution of dichloromethane (320ml) containing tert-butyldimethylchlorosilane (0.19mol) while maintaining the temperature at 20°C. After the dropwise addition, stir at room temperature for 8 hours. TLC (thin plate chromatography) detection showed that the reaction of the raw materials was basically complete, and the resulting solution was washed with 880 ml of purified water, and then dried and dehydrated with anhydrous sodium sulfate.
[0114] Add dimethylsulfoxide (0.096mol) and triethylamine (0.094mol) successively to the dehydrated solution, mix well and cool down to -30°C, add phenoxyphosphoryl dichloride (0.088mol) dropwise dichloromethane (25ml) solution, control the reaction temperature at -15°C...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com