Synthesis technology of 1-ethoxycarbonyl-5-methyl-(3R)-(tert-butyldimethylsilyloxy) glutaric ester
A technology of tert-butyldimethylsiloxyglutarate and ethoxycarbonyl, which is applied in the field of compound synthesis technology, can solve the problems of serious environmental pollution, complicated production process, and large discharge of three wastes, and achieve environmental friendliness, The effect of mild reaction conditions and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
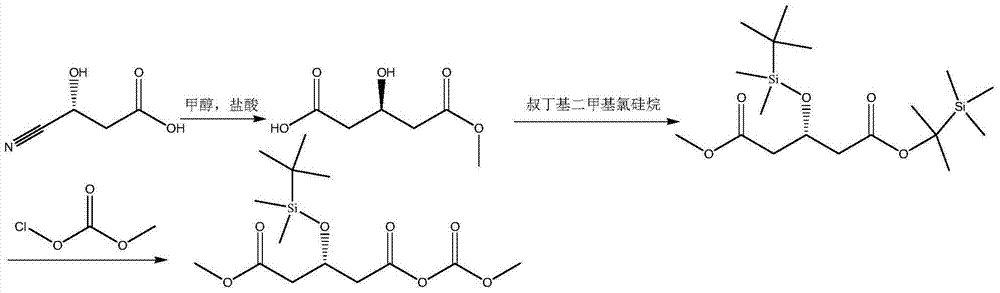
[0020] In a 1000ml three-necked flask, equipped with a thermometer and constant pressure dropping funnel and magnetic stirring. At room temperature, add 160.0 grams of methanol to the reaction flask, then add 115.0 grams of (R)-(-)-4-cyano-3-hydroxybutyric acid, cool to -5°C, and pass 73.0 grams of dry hydrogen chloride with stirring. React for 5 hours, extract with 500ml of dichloromethane, dry the extract with anhydrous sodium sulfate, filter, distill the filtrate to remove the solvent, and distill the residue under reduced pressure to obtain (R)-(+)-3-hydroxyglutarate monomethyl ester 129.6g, without separation, add 500ml of dichloromethane, 108.8g of imidazole, 240.0g of tert-butyldichlorosilane, react at 20℃ for 5 hours, add 100ml of 5% sodium bicarbonate aqueous solution after the reaction, organic phase Dry with anhydrous sodium sulfate, filter, and evaporate the filtrate to remove the solvent to obtain methyl tert-butyldimethylsilyl-(R)-3-[(tert-butyldimethylsilyl)oxy]g...
Embodiment 2
[0022] In a 1000ml three-necked flask, equipped with a thermometer and constant pressure dropping funnel and magnetic stirring. At room temperature, add 320 grams of methanol to the reaction flask, and then add 115.0 grams of (R)-(-)-4-cyano-3-hydroxybutyric acid. The temperature is lowered to 10°C, and 109.5 grams of dry hydrogen chloride is introduced under stirring. After 10 hours, extract with 500ml of dichloromethane, the extract phase was dried over anhydrous sodium sulfate, filtered, the filtrate was evaporated to remove the solvent, and the residue was distilled under reduced pressure to obtain (R)-(+)-3-hydroxyglutarate monomethyl ester 145.8 G, without separation, add 500ml of dichloromethane, 183.6g of imidazole, 405.0g of tert-butyldichlorosilane, react at 50℃ for 10 hours, add 100ml of 10% sodium bicarbonate aqueous solution after the reaction, and use for organic phase Dry with anhydrous sodium sulfate, filter, and evaporate the solvent from the filtrate to obtain...
Embodiment 3
[0024] In a 1000ml three-necked flask, equipped with a thermometer and constant pressure dropping funnel and magnetic stirring. At room temperature, add 300 grams of methanol to the reaction flask, and then add 115.0 grams of (R)-(-)-4-cyano-3-hydroxybutyric acid. The temperature is reduced to 0°C, and 100 grams of dry hydrogen chloride is introduced under stirring. After 8 hours, extract with 500ml of dichloromethane, the extract phase was dried over anhydrous sodium sulfate, filtered, the filtrate was evaporated to remove the solvent, and the residue was distilled under reduced pressure to obtain (R)-(+)-3-hydroxyglutarate monomethyl ester 127.5 Gram, without separation, add 500ml of dichloromethane, 180g of imidazole, 400g of tert-butyldichlorosilane, and react at 30℃ for 8 hours. After the reaction, add 100ml of 10% sodium bicarbonate aqueous solution, and use for organic phase. Dry with anhydrous sodium sulfate, filter, and evaporate the solvent from the filtrate to obtain...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap