Uvioresistant absorber used for pesticide preparation
A technology of anti-ultraviolet and pesticide preparations, applied in the application, pesticides, biocides and other directions, to achieve the effects of improving utilization, delaying photolysis, and improving control effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
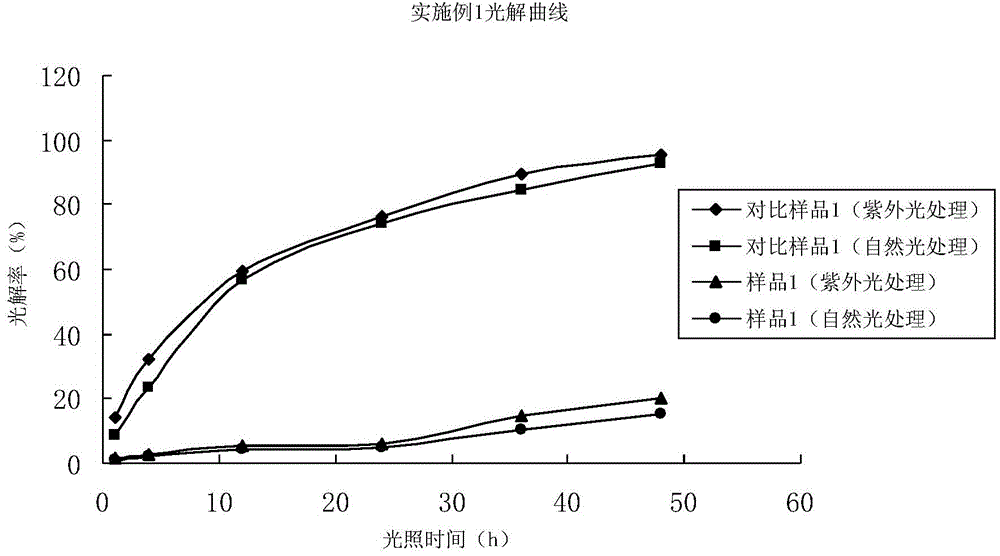
Embodiment 1
[0067] 2% emamectin emulsifiable concentrate (sample 1)
[0068] Put 2.3kg emamectin benzoate, 6kg tristyrylphenol polyoxyethylene ether, 0.01kg 2,6-dimethoxy-4-p-isothiazole carboxamide butenylphenol into the mixing kettle, 2kg dodecyl Calcium benzenesulfonate, 25kg dimethyl carbonate, 100kg made up of sec-butyl acetate, fully stirred, promptly got 2% emamectin emulsifiable concentrate (sample 1).
[0069] 2% emamectin emulsifiable concentrate (comparative sample 1)
[0070] In the mixing kettle, drop into 2.3kg emamectin benzoate, 6kg tristyrylphenol polyoxyethylene ether, 2kg calcium dodecylbenzenesulfonate, 25kg dimethyl carbonate, and sec-butyl acetate make up 100kg, fully stir evenly, namely Obtain 2% emamectin emulsifiable concentrate (comparative sample 1).
[0071] The above two samples were processed according to the test method and steps of the present invention, and finally obtained 1h, 4h, 12h, 24h, 36h, 48h of each time period of darkroom, ultraviolet light and...
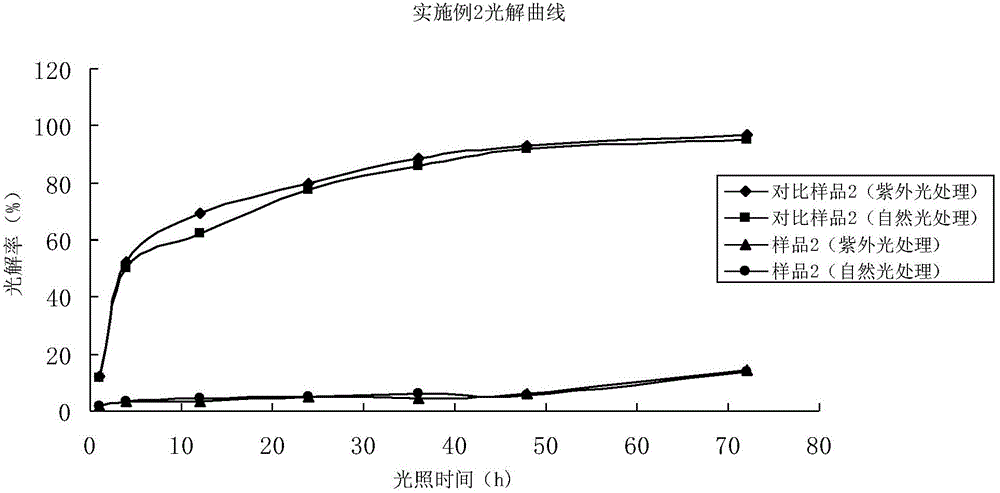
Embodiment 2
[0090] 3.2% Abamectin EC (sample 2)
[0091] Put 3.2kg of abamectin emulsifiable concentrate into the mixing tank, pump in 25kg of ethyl acetate, stir to complete the dissolution of abamectin, and then put in 0.05kg of 2,6-dimethoxy-4-p-isothiazole carboxamide butene Phenylphenol, 6kg nonylphenol polyoxyalkylene ether, trimethylbenzene make up to 100kg, fully stir, obtain 3.2% abamectin emulsifiable concentrate (sample 2).
[0092] 3.2% Abamectin EC (comparative sample 2)
[0093] Add 3.2kg abamectin emulsifiable concentrate into mixing, stir after sucking in 25kg ethyl acetate and finish dissolving abamectin, then drop into 6kg nonylphenol polyoxyalkylene ether, trimethylbenzene to make up to 100kg, fully stir, get 3.2 % Abamectin EC (comparative sample 2).
[0094] The above-mentioned two samples are processed according to the test method and steps of the present invention, and finally obtain darkroom, ultraviolet light and natural light irradiation treatment samples of ea...
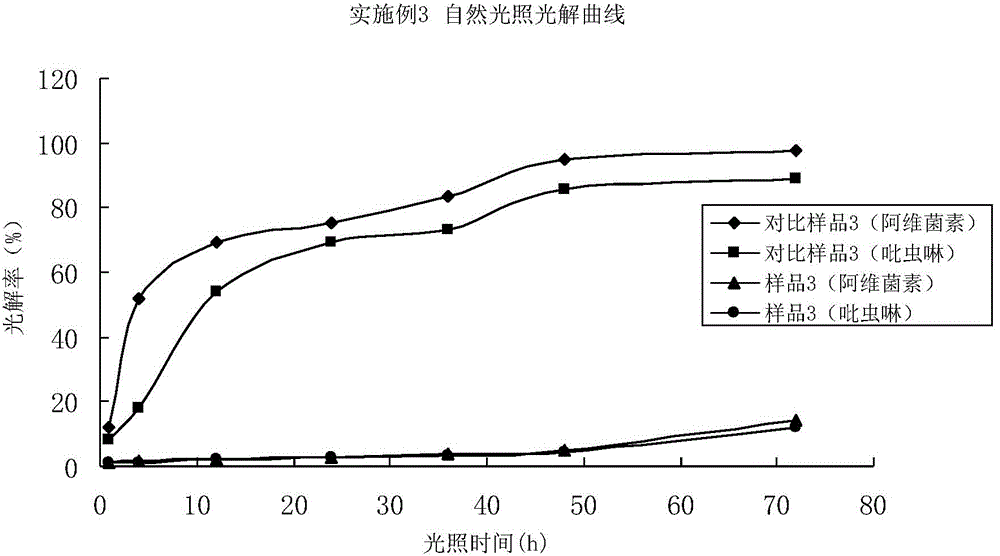
Embodiment 3
[0114] 2.2% Avi imidacloprid EC (sample 3)
[0115] Put 0.2kg abamectin and 2.2kg imidacloprid into the mixing tank, pump in 15kg N-methylpyrrolidone, stir to dissolve abamectin and imidacloprid completely, and then put 0.1kg 2,6-dimethoxy-4-para Isothiazole carboxamide butenylphenol, 8kg tristyrylphenol formaldehyde resin polyoxyethylene ether, 2kg fatty alcohol polyoxyethylene ether, trimethylbenzene make up to 100kg. Stir evenly to get 2.2% Avi imidacloprid emulsifiable concentrate (sample 3)
[0116] 2.2% Avi imidacloprid emulsifiable concentrate (comparative sample 3)
[0117]Drop into 0.2kg abamectin, 2.2kg imidacloprid in the mixing kettle, suck in 15kgN-methylpyrrolidone, stir to dissolve abamectin and imidacloprid completely, then drop into 8kg tristyryl phenol formaldehyde resin polyoxyethylene ether, 2kg of fatty alcohol polyoxyethylene ether and trimethylbenzene make up to 100kg. Stir evenly to get 2.2% Avi imidacloprid emulsifiable concentrate (sample 3)
[01...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com