A polysaccharide nucleic acid pharmaceutical composition for treating infantile eczema
A technology for polysaccharide nucleic acid and pediatric eczema, applied in the field of medicine, can solve the problems of heavy family burden and poor compliance of children, and achieve the effects of improving compliance, prolonging dosing interval, and improving treatment effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045]
[0046]
[0047] (1) Dissolving BCG polysaccharide nucleic acid and oxymatrine in 1 ml of PBS buffer solution containing trehalose and half of Tween 80 to make an aqueous phase solution (W1);
[0048] (2) Lactide / glycolide copolymer is dissolved in 4 milliliters of dichloromethane to make oil phase solution (O);
[0049] (3) Add the above-mentioned water phase solution (W1) into the oil phase solution (O), make the two solutions stir and mix at a temperature of 25°C, a stirring speed of 8000 rpm, and a stirring time of 0.05 hours to prepare Colostrum W1 / O, stored at 4-10°C;
[0050] (4) Add the above-mentioned colostrum W1 / O into 300ml of polyvinyl alcohol under the condition of stirring at 1500 rpm, the stirring temperature is 15°C, and the stirring time is 0.2 hours to make double milk W1 / O / W2. Store at 4-10°C;
[0051] (5) Add the double emulsion W1 / O / W2 to 1% NaCl aqueous solution of 3 times the volume of the double emulsion, stir at 1500 rpm to volatilize ...
Embodiment 2
[0054]
[0055] (1) Dissolving BCG polysaccharide nucleic acid and oxymatrine in 2 ml of PBS buffer solution containing trehalose and half of Tween 80 to make an aqueous phase solution (W1);
[0056] (2) Lactide / glycolide copolymer was dissolved in 6 milliliters of dichloromethane to make oil phase solution (O);
[0057] (3) Add the above water phase solution (W1) into the oil phase solution (O), make the two solutions stir and mix at a temperature of 25°C, a stirring speed of 8000 rpm, and a stirring time of 0.2 hours to prepare Colostrum W1 / O, stored at 4-10°C;
[0058] (4) Add the above-mentioned colostrum W1 / O into 400ml of polyvinyl alcohol under the condition of stirring at 1500 rpm, the stirring temperature is 15°C, and the stirring time is 0.2 hours to make double milk W1 / O / W2. Store at 4-10°C;
[0059] (5) Add the double emulsion W1 / O / W2 into 1% NaCl aqueous solution of 4 times the volume of the double emulsion, stir at 1500 rpm to volatilize the organic solvent,...
Embodiment 3
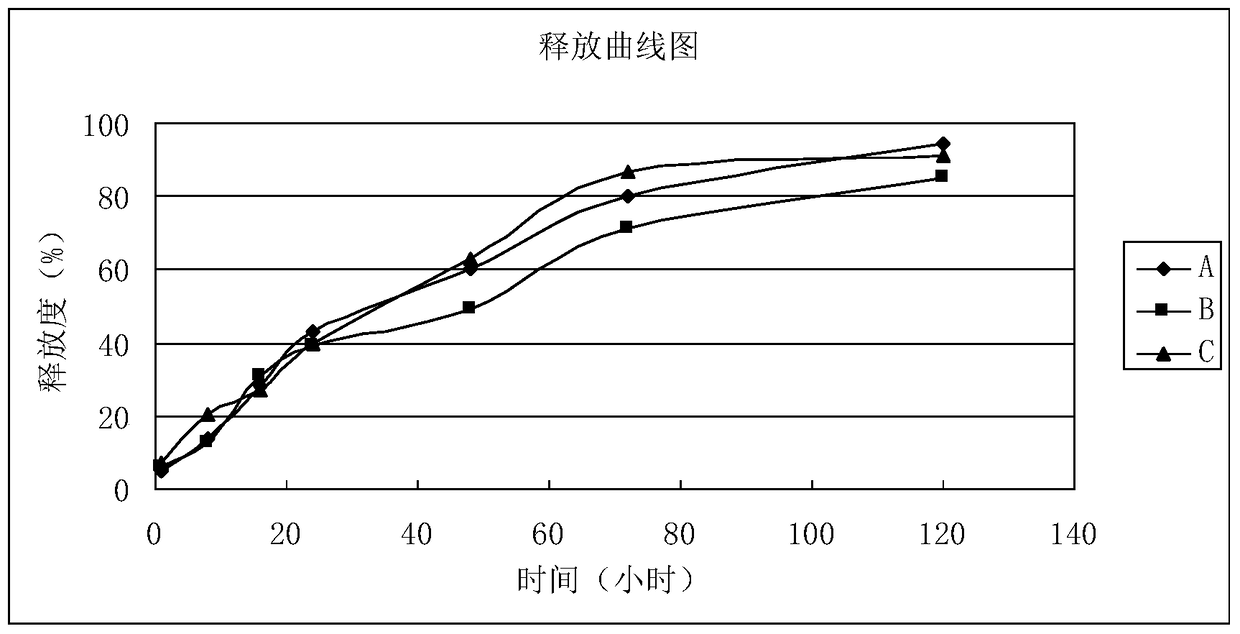
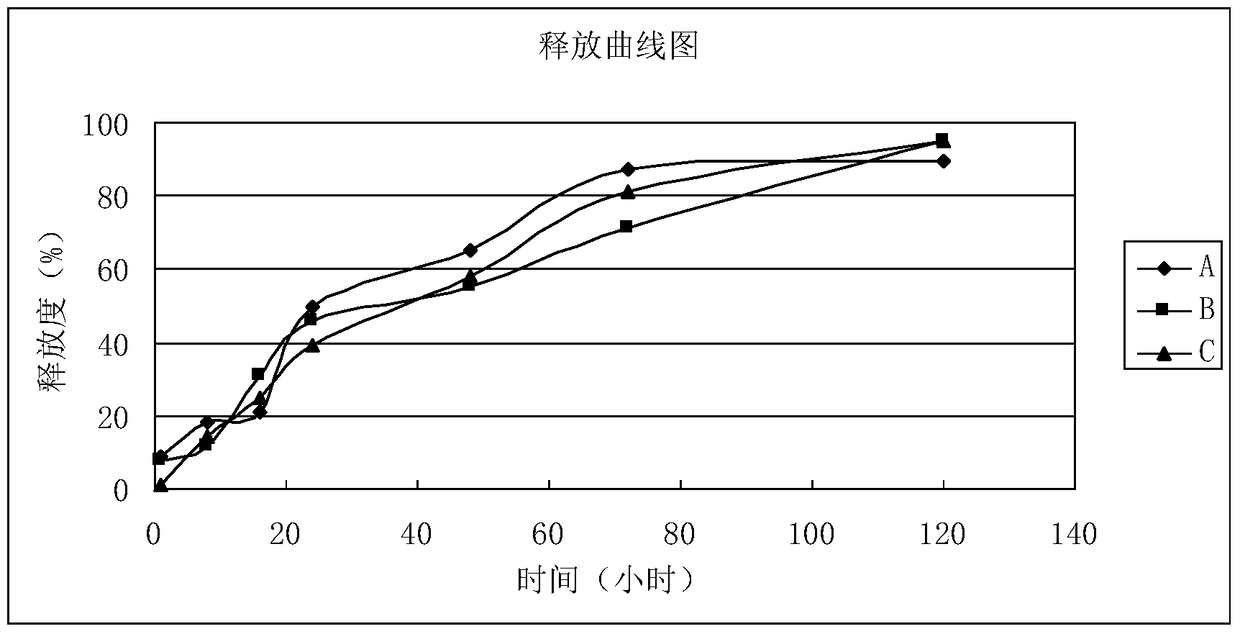
[0061] The in vitro release test of embodiment 3 microspheres
[0062] Test sample: microspheres prepared according to the method described in Example 1-2 of the present invention.
[0063] Experimental equipment: water bath constant temperature oscillator, centrifuge.
[0064] Experimental conditions: temperature: 37±0.5°C, rotation speed: 100rpm.
[0065] Experimental method: Accurately weigh about 10 mg of the experimental sample, place it in a clear bottle with a cap of 100 ml in volume, add 90 ml of release medium (0.02% Tween-80), place it in a constant temperature oscillator in a water bath, and maintain a certain temperature and rotation speed Take samples on time.
[0066] Sampling method: extract 5ml of solution, centrifuge at 3000rpm for 10min, add 5ml of release medium, and use HPLC to detect the solution.
[0067] Sampling time points (hours): 1, 8, 16, 24, 48, 3 days, 5 days.
[0068] Test results: the microspheres prepared in Example 1 and Example 2 of the p...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| cumulative release rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com