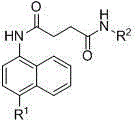
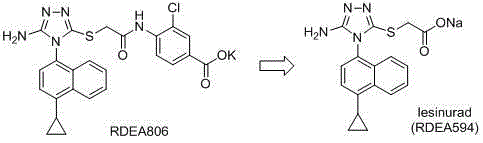
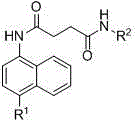
Nitronaphthalene-ring-containing butanedioic acid amide derivatives, preparing method thereof and uses of the derivatives
A technology of alkyl and compounds, applied in the field of uric acid transporter 1 inhibitors, can solve problems such as fulminant hepatitis, allopurinol liver and bone marrow toxicity, and allergies
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] .
[0030] A. Compounds IV-1 Synthesis
[0031] 3.76g (20 mmol) compound II-1 and 2.64 g (20 mmol) of compound III Dissolve in 50 mL of dry THF, stir under ice-water bath cooling, add 4.13 g (20 mmol) of dicyclohexylcarbodiimide (DCC) and 0.61 g (5 mmol) of 4-dimethylaminopyridine (DMAP), and then Stir at room temperature until the reaction is complete (within 12 h) as detected by TLC. The reaction mixture was poured into 300 mL of ice water, stirred, using 100 mL × 3 CH 2 Cl 2 Extract, combine the extract phases, successively wash with 100 mL of 1% dilute hydrochloric acid and 100 mL of 5% brine, and dry over anhydrous sodium sulfate. The desiccant was removed by suction filtration, the filtrate was evaporated to dryness on a rotary evaporator, and the obtained residue was purified by column chromatography to obtain compound IV-1 , white solid, ESI-MS, m / z = 325([M+Na] + ).
[0032] B. Compounds V-1 Synthesis
[0033] compound IV-1 Dissolve 4.53 ...
Embodiment 2-13
[0038] With reference to the operating steps of Example 1, the compounds listed in the following table were prepared:
[0039]
Embodiment 9
[0041] The compounds of the present invention and related compounds inhibit IC of URAT1 50 The values are determined in a similar manner as described in the literature (Example 12 in US2014 / 0005136). The results are listed below.
[0042]Construction of a cell line stably expressing the humanized URAT1 transporter: The humanized URAT1 gene (SLC22A112) was subcloned from the plasmid pCMV6-XL-5 (Origene) into the eukaryotic expression plasmid pCMV6 / neo (Origene). Gene sequencing confirmed that the humanized URAT1 was consistent with the information recorded in the gene bank (NM_144585.2). HEK293 human embryonic kidney cells (ATCC# CRL-1573) were cultured in EMEM tissue culture medium under 5% CO 2 and 95% air atmosphere. pCMV6 / Neo / URAT1 was transfected onto HEK293 cells using L2000 type transfection reagent (Invitrogene). After 24 hours, the transfected cells were divided into 10 cm diameter tissue culture dishes and grown for another day, after which the medium was replac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com