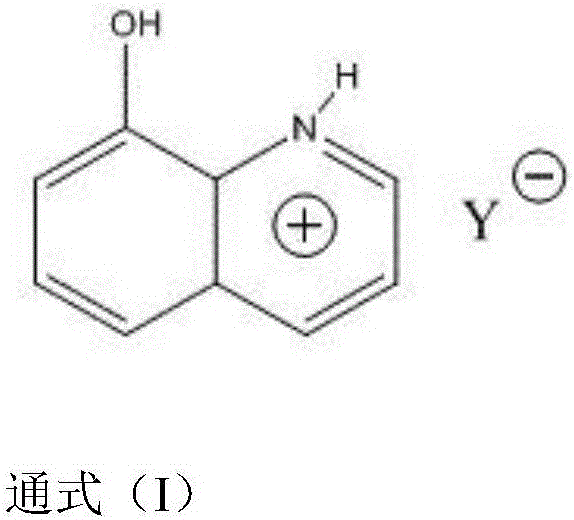
Preparation of 8-hydroxyquinoline thermosensitive ionic liquid and method for catalyzing long-chain fatty acid ethyl esterification
A technology of ionic liquids and hydroxyquinoline, applied in chemical instruments and methods, preparation of organic compounds, catalytic reactions, etc., can solve the problems of narrow application range, complicated preparation process, high cost, etc., and achieve wide application range and high catalytic efficiency , Ease of recycling
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Embodiment 1 (synthesis of ionic liquid): preparation method 1 of 8-hydroxyquinoline bisulfate ionic liquid
[0037] Add 0.1mol of 8-hydroxyquinoline into a round bottom flask, add 50mL of ethanol as a reaction solvent, cool down to 5°C in an ice bath, slowly add 0.09mol of concentrated sulfuric acid dropwise with a constant pressure titration funnel, drop it in half an hour, then Down at room temperature and stirred for 3h, a light yellow turbid liquid was obtained, and the reaction was terminated. The product was rotovaped and the ethanol was removed to give a pale yellow solid. Then wash with acetone for 3 to 4 times, and finally use absolute ethanol to recrystallize the crude product, and obtain a light yellow powder solid after vacuum drying, which is the product 8-hydroxyquinoline hydrogen sulfate.
Embodiment 2
[0038] Embodiment 2 (synthesis of ionic liquid): preparation method 2 of 8-hydroxyquinoline bisulfate ionic liquid
[0039] Add 0.1mol of 8-hydroxyquinoline into a round bottom flask, add 50mL of ethanol as the reaction solvent, cool down to 5°C in an ice bath, slowly add 0.1mol of concentrated sulfuric acid dropwise with a constant pressure titration funnel, drop it in half an hour, then Down at room temperature and stirred for 2h, a light yellow turbid liquid was obtained, and the reaction stopped. The product was rotovaped and the ethanol was removed to give a pale yellow solid. Then wash with acetone for 3 to 4 times, and finally use absolute ethanol to recrystallize the crude product, and obtain a light yellow powder solid after vacuum drying, which is the product 8-hydroxyquinoline hydrogen sulfate.
Embodiment 3
[0040] Embodiment 3 (synthesis of ionic liquid): preparation method 3 of 8-hydroxyquinoline bisulfate ionic liquid
[0041] Add 0.09mol of 8-hydroxyquinoline into a round bottom flask, add 50ml of ethanol as a reaction solvent, cool down to 3°C in an ice bath, slowly add 0.1mol of concentrated sulfuric acid dropwise with a constant pressure titration funnel, drop it in half an hour, then Stir at room temperature for 1.5h to obtain a light yellow turbid liquid, and the reaction stops. The product was rotovaped and the ethanol was removed to give a pale yellow solid. Then wash with acetone for 3 to 4 times, and finally use absolute ethanol to recrystallize the crude product, and obtain a light yellow powder solid after vacuum drying, which is the product 8-hydroxyquinoline hydrogen sulfate.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com