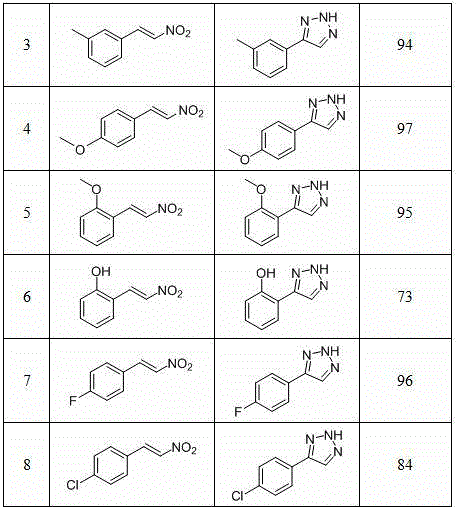
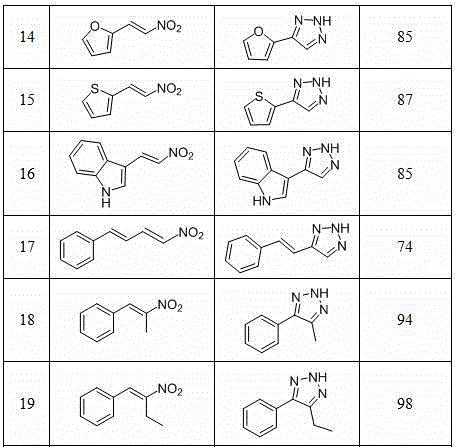
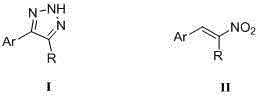Method for synthesizing NH-1,2,3-triazole
A synthesis method and azide technology, applied in organic chemistry and other directions, can solve the problems of harsh conditions, many reaction steps, and difficult to obtain raw materials, and achieve the effects of mild reaction conditions, low production cost, and wide application range.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Example 1: Preparation of 4-phenyl-N by β-nitrostyrene H -1,2,3-Triazole
[0019]
[0020] To a 250 mL round bottom flask, add β-nitrostyrene (10.5 g, 70 mmol), NaN 3 (5.5g, 84mmol) and 100mL N,N-dimethylformamide, then added p-toluenesulfonic acid (3.3g, 21mmol), heated to 60 ℃ in the air to react, the reaction was detected by thin layer chromatography, and the reaction After completion, it was cooled to room temperature, the reaction mixture was extracted with ethyl acetate and water, the organic phases were combined and spin-dried in vacuo, the reaction crude product was separated by recrystallization or column chromatography to obtain 9.4g of pure product, the product was a white solid, and the yield 93%.
[0021] structural analysis
[0022] 1 H NMR (DMSO- d 6 , 400 MHz), 1 drop TFA : δ 15.19 (s, 1 H), 8.35 (s, 1 H), 7.87 (d, J = 6.8 Hz, 2 H), 7.44 (d, J = 7.2 Hz, 2 H ), 7.35 (d, J = 6.8 Hz, 1 H); 13 C NMR (DMSO- d 6 , 100 MHz) , 1 drop TFA: δ 145.3, ...
Embodiment 2
[0024] Example 2: Preparation of 4-methoxyphenyl-N by β-nitro-p-methoxystyrene H -1,2,3-Triazole
[0025]
[0026] To a 10mL round bottom flask, add β-nitro-p-methoxystyrene (53.7 mg, 0.3mol), NaN 3 (29.3 mg, 0.45mmol) and 3mL N,N-dimethylformamide, then added p-toluenesulfonic acid (25.8 mg, 0.15mmol), heated to 60°C in air to react, and detected the reaction by thin layer chromatography, After the reaction was completed, it was cooled to room temperature, the reaction mixture was extracted with ethyl acetate and water, the organic phases were combined and spin-dried in vacuo, the reaction crude product was separated by recrystallization or column chromatography to obtain 50.9 mg of pure product, the product was a white solid, Yield 97%.
[0027] Structural analysis: 1 H NMR (DMSO- d 6 , 400 MHz), 1 drop TFA: δ 8.22 (s, 1 H), 7.79 (d, J = 8.4 Hz, 2 H), 7.01 (d, J = 8.8 Hz, 2 H), 3.77 (s, 3 H ). 13 C NMR (DMSO- d 6 , 100 MHz), 1 drop TFA: δ 159.4, 144.9, 127.1, 12...
Embodiment 3
[0029] Example 3: Preparation of 2,4-dichlorophenyl-N from β-nitro 2,4-dichlorostyrene H -1,2,3-Triazole
[0030]
[0031] To a 10 mL round bottom flask, add β-nitro 2,4-dichlorostyrene (65.1 mg, 0.3 mmol), NaN 3 (29.3mg, 0.45mmol) and 3mL N,N-dimethylformamide, then added p-toluenesulfonic acid (25.8 mg, 0.15mmol), heated to 60°C in air to react, and detected the reaction by thin layer chromatography, After the reaction was completed, it was cooled to room temperature, and the reaction mixture was extracted with ethyl acetate and water. The organic phases were combined and spin-dried in vacuo. The reaction crude product was separated by recrystallization or column chromatography to obtain 57.5 mg of pure product. The product was a white solid. Yield 90%.
[0032] Structural analysis: 1 H NMR (DMSO- d 6 , 400 MHz), 1 drop TFA: δ 8.41 (s, 1 H), 7.96 (d, J = 8.0 Hz, 1 H), 7.72 (s, 1 H), 7.52 (d, J = 8.4 Hz, 1 H ). 13 C NMR (DMSO- d 6 , 100 MHz), 1 drop TFA: δ 141.6,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com