Pleuromutilin derivative, and preparation method and application thereof
A technology of pleuromutilin and derivatives, which is used in pharmaceutical formulations, medical preparations containing active ingredients, antibacterial drugs, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0037]Preferred embodiments of the present invention are described below, and it should be understood that the preferred embodiments described here are only used to illustrate and explain the present invention, and are not intended to limit the present invention.
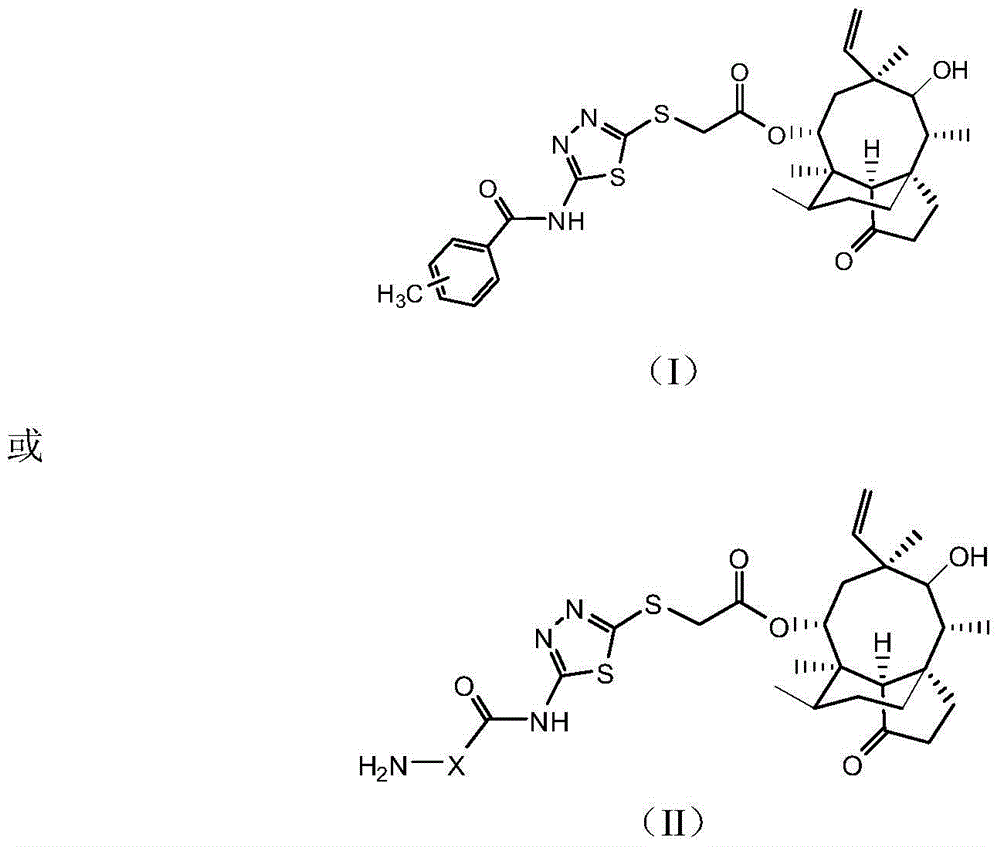
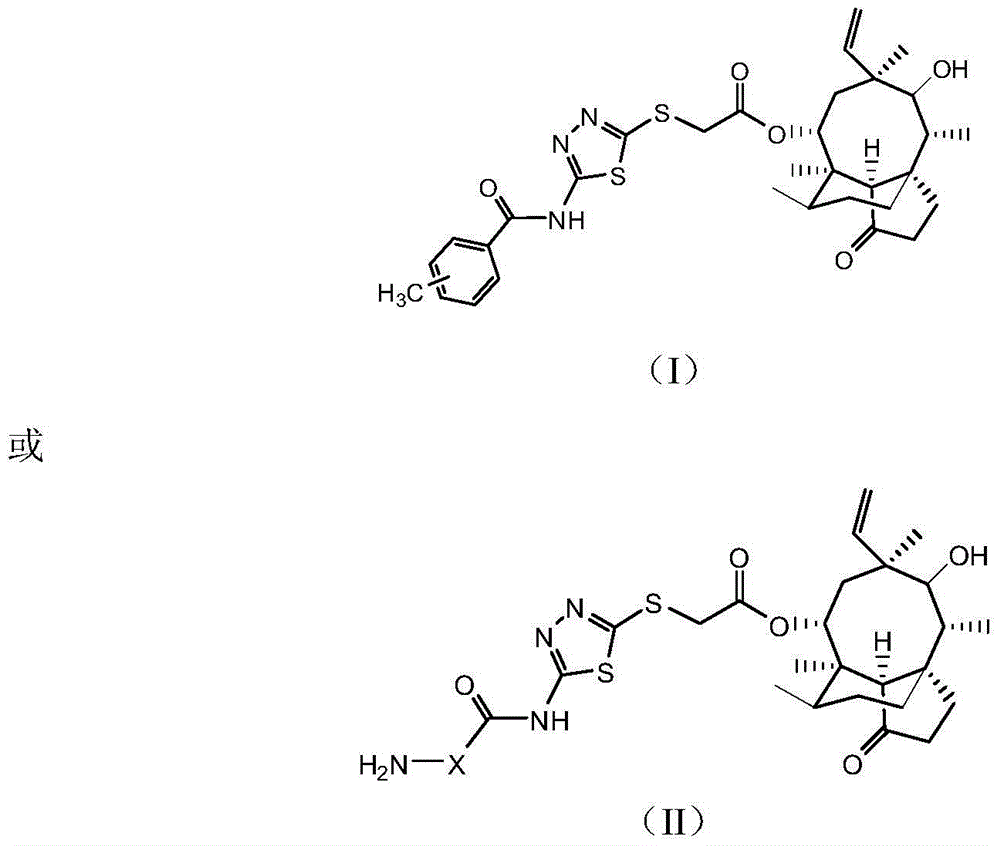
[0038] Pleuromutilin derivatives, chemical structural formula such as I or II:
[0039]
[0040] Wherein: wherein: in formula (II) H2N-X is One of.
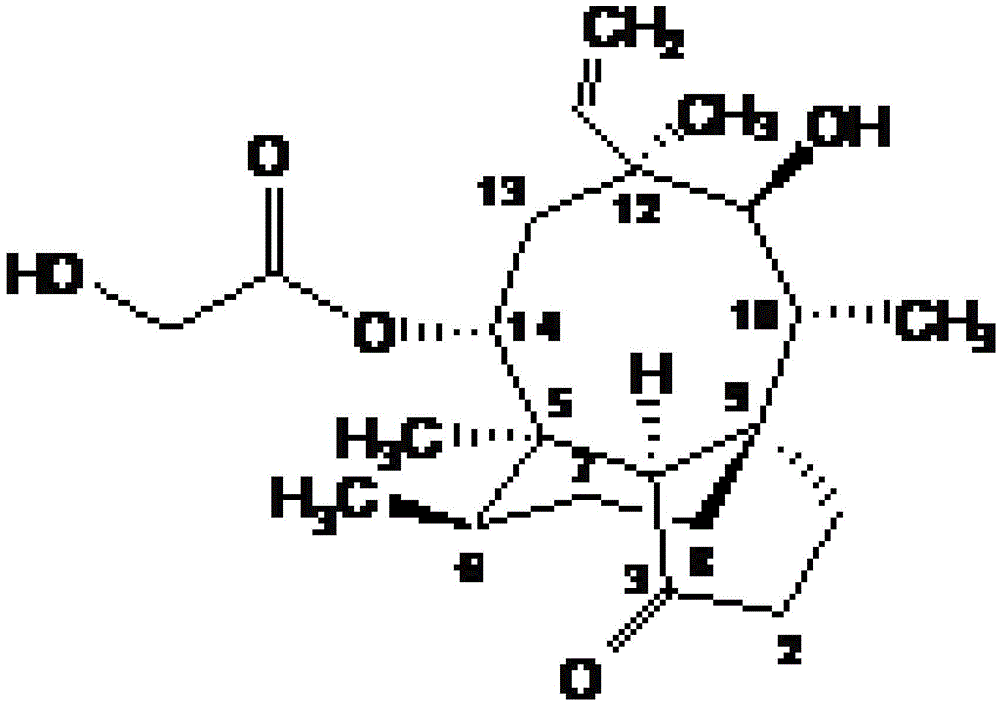
[0041] A kind of method for preparing above-mentioned compound, at first synthesizing intermediate-14-O-[(2-amino-1,3,4,-thiadiazol-5 base) mercaptoacetyl] Mutilin (compound 4), then Using this intermediate as a raw material, the above-mentioned compounds 5a-c, 6a-d were synthesized, respectively. The reaction scheme is as follows:
[0042]
[0043] Synthesis of 22-O-(4-toluenesulfonyl)oxyacetylmtilin
[0044] 75.7g (0.2mol) of pleuromutilin and 42g (0.22mol) of p-toluenesulfonyl chloride were dissolved in 200ml of methyl tert-butyl ether, and the mixture ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com