A kind of conjugated polymer containing sulfone group and its preparation method and application
A polymer, sulfone-based technology, applied in novel conjugated polymers and preparation methods thereof, and in the field of organic optoelectronics, can solve problems such as inefficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
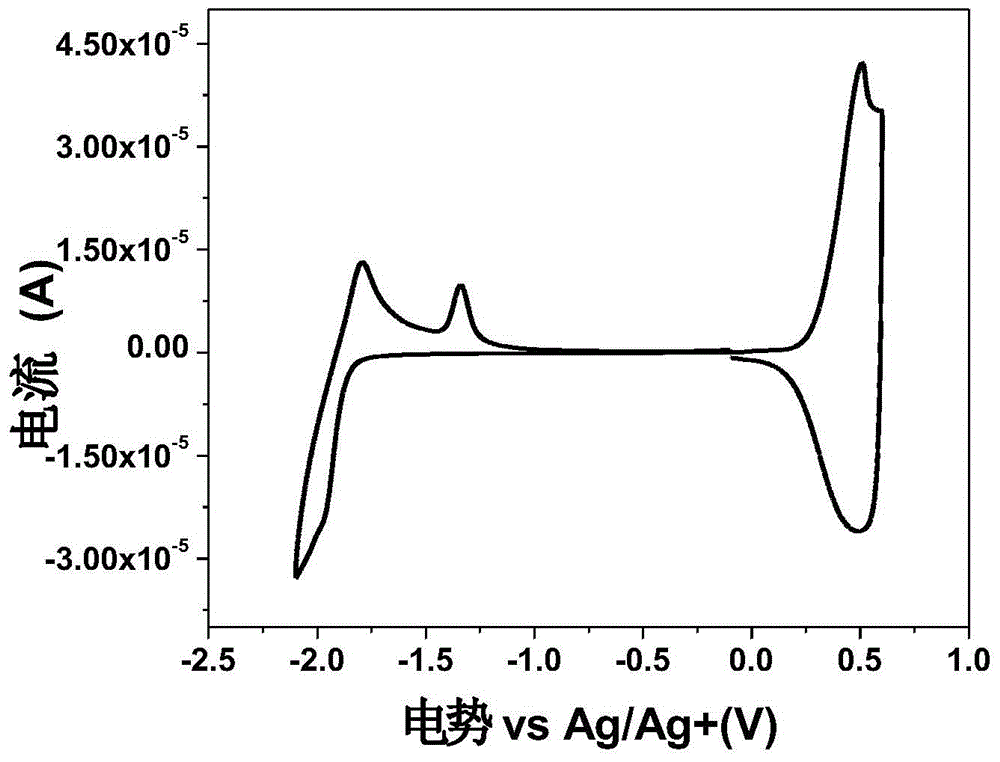
Embodiment 1
[0108] Poly{[4,8-bis((2-ethyl-hexyl)sulfonylthienyl)-benzo[1,2-b:4,5-b']dithiophene]-co[(2,5) -Dithiophene bi[thienyl[3,4-b]thiophene-2 base-]-2-carboxylic acid-2-octyldodecyl-1-ester]} (polymer P1) synthetic method 1
[0109] The chemical reaction flow diagram of polymer P1 is as follows Figure 19 Shown, concrete reaction steps and reaction conditions are as follows:
[0110] Take the polymer PBDTTSDTTT-E (such as Figure 19 Shown) 0.2mmol, it was dissolved in 20ml of chloroform solvent and placed in an ice-water bath and stirred for 30 minutes, then added 10% acetic acid. With rapid stirring, 1 ml of 30% aqueous hydrogen peroxide was added dropwise. After maintaining the temperature and stirring rapidly for 2 hrs, return to room temperature and add 1 ml of 30% hydrogen peroxide solution dropwise and stir for 12 hrs to stop the reaction. The reacted polymer solution was sequentially washed with sodium thiosulfate aqueous solution and deionized water. The extracted pol...
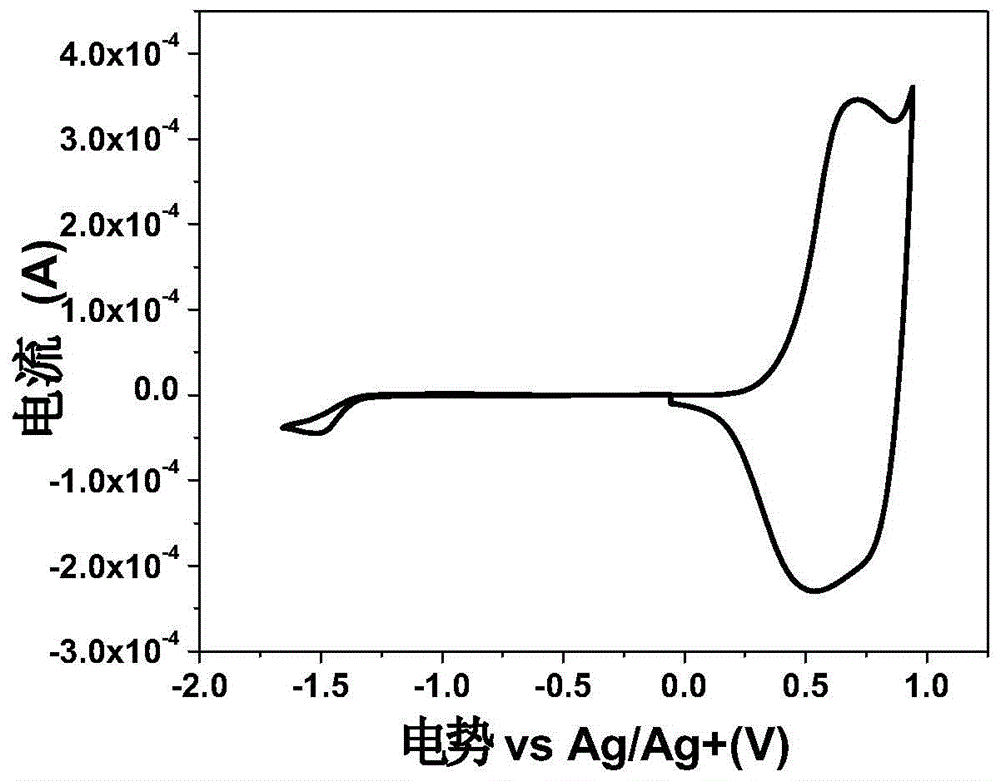
Embodiment 2
[0112] Poly{[4,8-bis((2-ethyl-hexyl)sulfonylthienyl)-benzo[1,2-b:4,5-b']dithiophene]-co[(2,5) -Dithiophene bi[thienyl[3,4-b]thiophene-2 base-]-2-carboxylic acid-2-octyl dodecyl ester]} (polymer P1) synthetic method 2
[0113] The chemical reaction flow diagram of polymer P1 is as follows Figure 20 Shown, concrete reaction steps and reaction conditions are as follows:
[0114] Take the polymer PBDTTSDTTT-E (such as Figure 20 Shown) 0.2mmol, it was dissolved in 30ml chloroform solvent and placed in an ice-water bath and stirred for 30 minutes. Under rapid stirring, 15 ml of 1.2 mmol m-chlorobenzoic acid in chloroform were added dropwise. Keep the temperature and stir rapidly for 12hr to stop the reaction. The reacted polymer solution was sequentially washed with sodium thiosulfate aqueous solution and deionized water. The extracted polymer solution was slowly immersed in methanol (50 mL), and the precipitated solid polymer was sequentially eluted with methanol and n-hex...
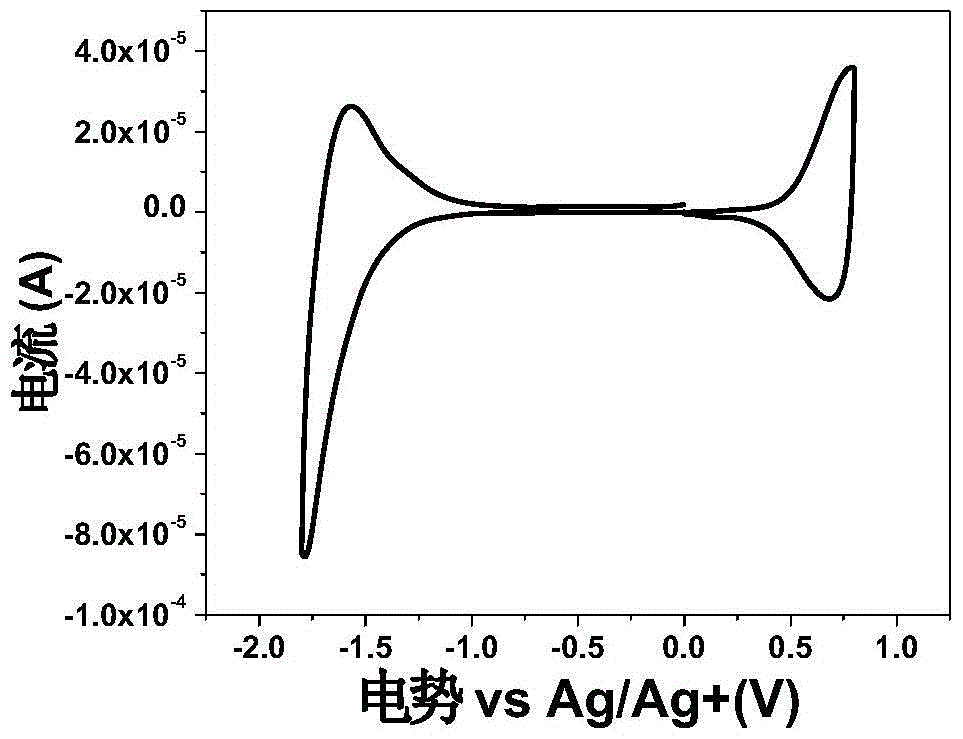
Embodiment 3
[0116] Embodiment 3 (elemental analysis test of polymer of the present invention)
[0117] Table 1 Theoretical and measured element content data of polymer PBDTTSDTTT-E and polymer P1
[0118] polymer
Carbon content (%)
Hydrogen content (%)
PBDTTSDTTT-E (Theory)
65.35
6.84
Polymer P1 (Theoretical)
62.21
6.51
Polymer P1(H 2 o 2 )
62.51
6.56
Polymer P1 (MCPBA)
59.92
6.21
[0119] The errors between the measured carbon and hydrogen contents of polymer P1 in Example 1 and the theoretical values are 0.48% and 0.77%, respectively. The results are within the error range, and the results of this reaction are credible.
[0120] The error between the measured carbon and hydrogen content of polymer P1 in Example 2 and the theoretical value is 3.68% and 4.61%, respectively. The result shows that there is some peroxidation, but it is also within the acceptable range, and the result of this reaction is ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| dispersity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com