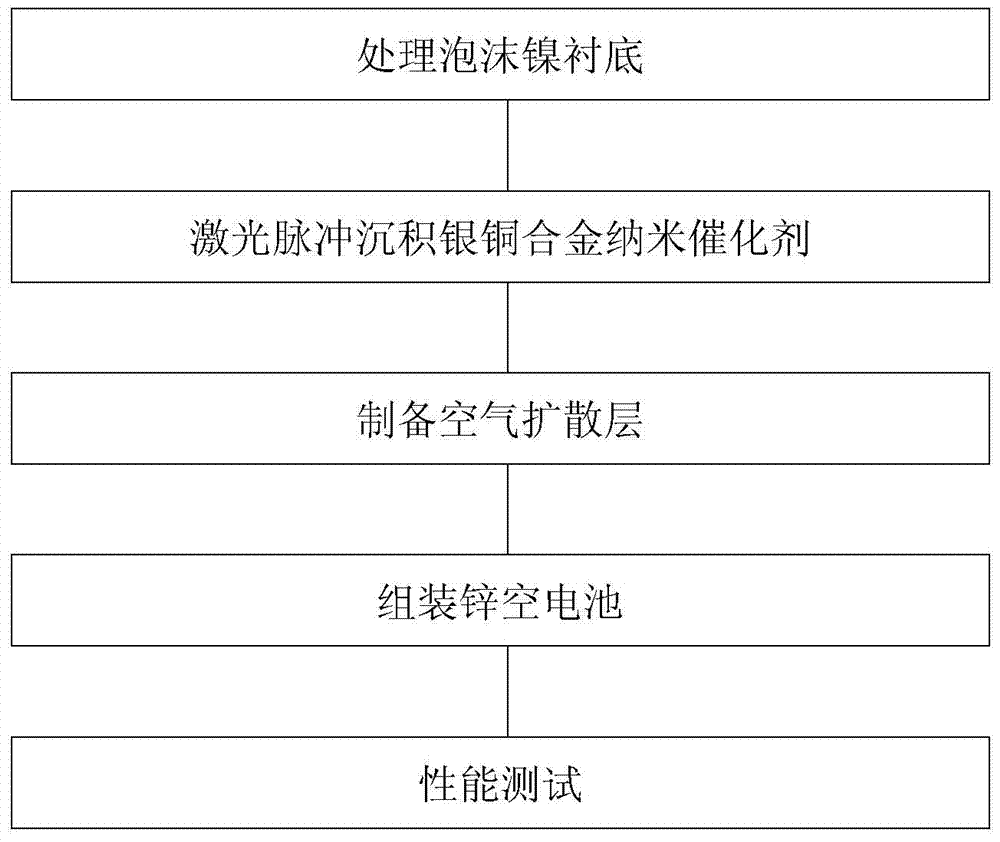
Silver-copper nano-alloy air electrode catalyst layer and deposition method thereof
A nano-alloy, air electrode technology, applied in battery electrodes, circuits, electrical components, etc., can solve problems such as the shedding of Ag-based catalytic active components, weak catalytic activity of oxygen reduction reaction, and reduced catalytic activity of air electrodes.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] This embodiment is a silver-copper nano-alloy air electrode catalyst layer.
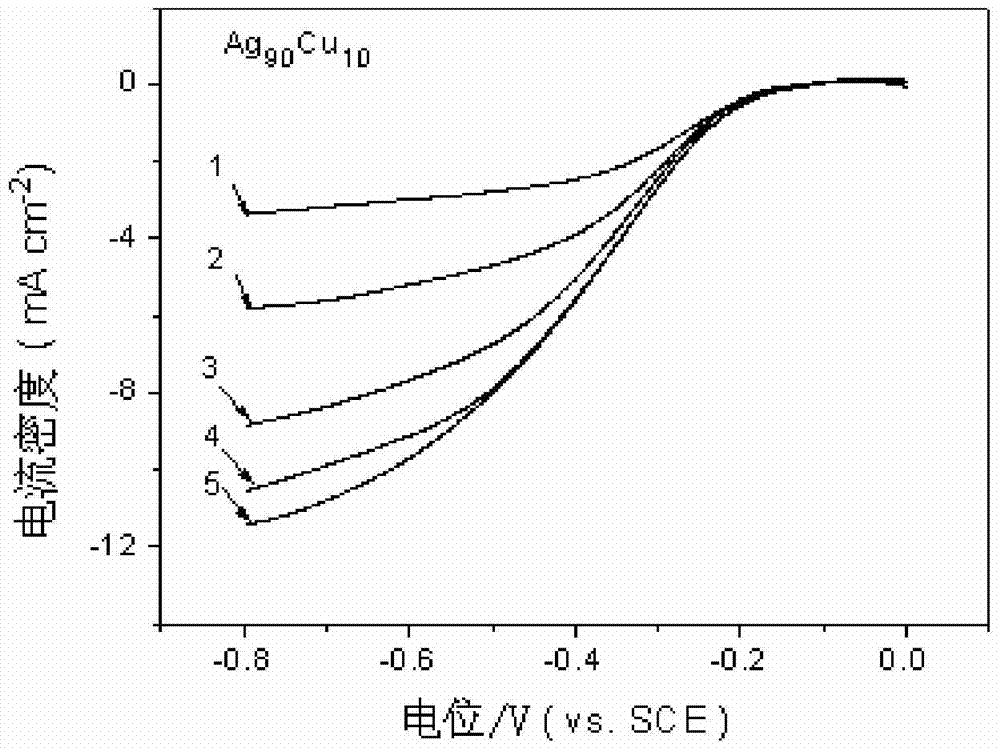
[0030] The silver-copper nano-alloy air electrode catalyst layer is a thin film composed of 50-90% Ag and 10-50% Cu, and the stated percentages are atomic percentages. In this embodiment, the atomic percentage of Ag is 90%, and the atomic percentage of Cu is 10%. Through transmission electron microscope observation, the microstructure of the silver-copper nano-alloy air electrode catalyst layer is silver-copper nano-alloy particles dispersed in the copper amorphous matrix, the average particle size of the nanoparticles is 2.8nm, and the distribution particle size is between 1 and 5nm. between.
[0031] This embodiment also proposes a method for preparing the silver-copper nano-alloy air electrode catalyst layer, the specific process is:
[0032] Step 1, processing the nickel foam substrate. Take nickel foam with a thickness of 1 mm and soak it in acetone for 3 hours to remove oil, soak it i...
Embodiment 2
[0040] This embodiment is a silver-copper nano-alloy air electrode catalyst layer.
[0041] The silver-copper nano-alloy air electrode catalyst layer is a thin film composed of 50-90% Ag and 10-50% Cu, and the stated percentages are atomic percentages. In this embodiment, the atomic percentage of Ag is 75% and the atomic percentage of Cu is 25%. Through transmission electron microscope observation, the microstructure of the silver-copper nano-alloy air electrode catalyst layer is silver-copper nano-alloy particles dispersed in the copper amorphous matrix, the average particle size of nanoparticles is 2.5nm, and the distribution particle size is between 1 and 5nm. between.
[0042] This embodiment also proposes a method for preparing the silver-copper nano-alloy air electrode catalyst layer, the specific process is:
[0043] Step 1, processing the nickel foam substrate. Take nickel foam with a thickness of 1 mm and soak it in acetone for 3 hours to remove oil, soak it in 5% ...
Embodiment 3
[0051] This embodiment is a silver-copper nano-alloy air electrode catalyst layer.
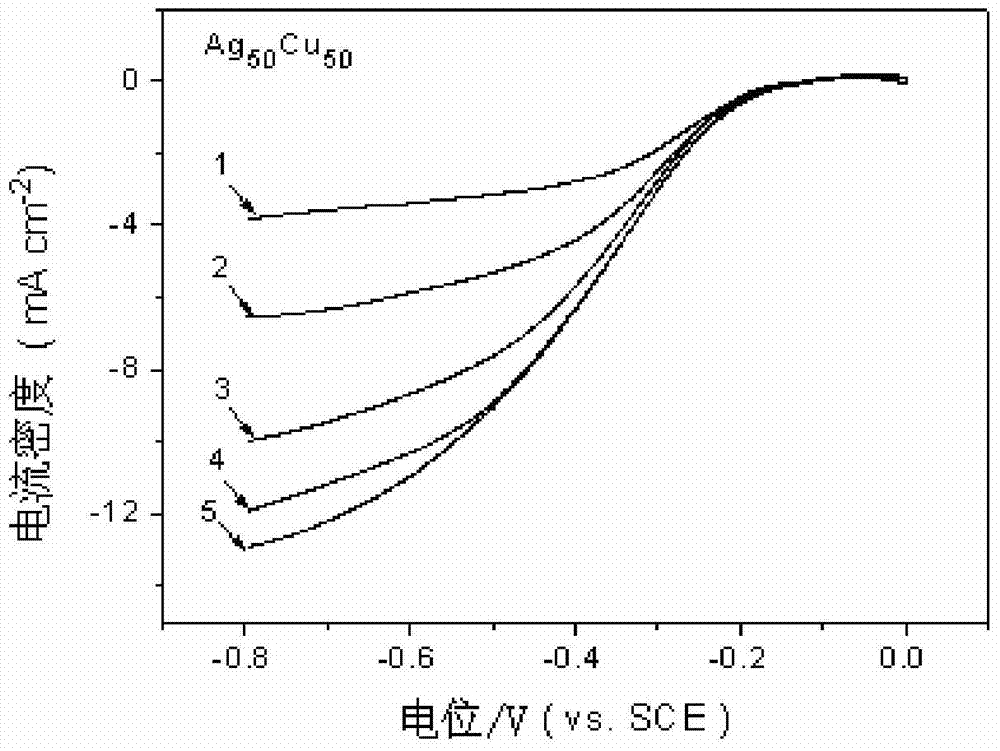
[0052] The silver-copper nano-alloy air electrode catalyst layer is a thin film composed of 50-90% Ag and 10-50% Cu, and the stated percentages are atomic percentages. In this embodiment, the atomic percentage of Ag is 50%, and the atomic percentage of Cu is 50%. Through transmission electron microscope observation, the microstructure of the silver-copper nano-alloy air electrode catalyst layer is silver-copper nano-alloy particles dispersed in the copper amorphous matrix, the average particle size of the nanoparticles is 2.6nm, and the distribution particle size is between 1 and 5nm. between.
[0053] This embodiment also proposes a method for preparing the silver-copper nano-alloy air electrode catalyst layer, the specific process is:
[0054] Step 1, processing the nickel foam substrate. Take nickel foam with a thickness of 1 mm and soak it in acetone for 3 hours to remove oil, soak it i...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com