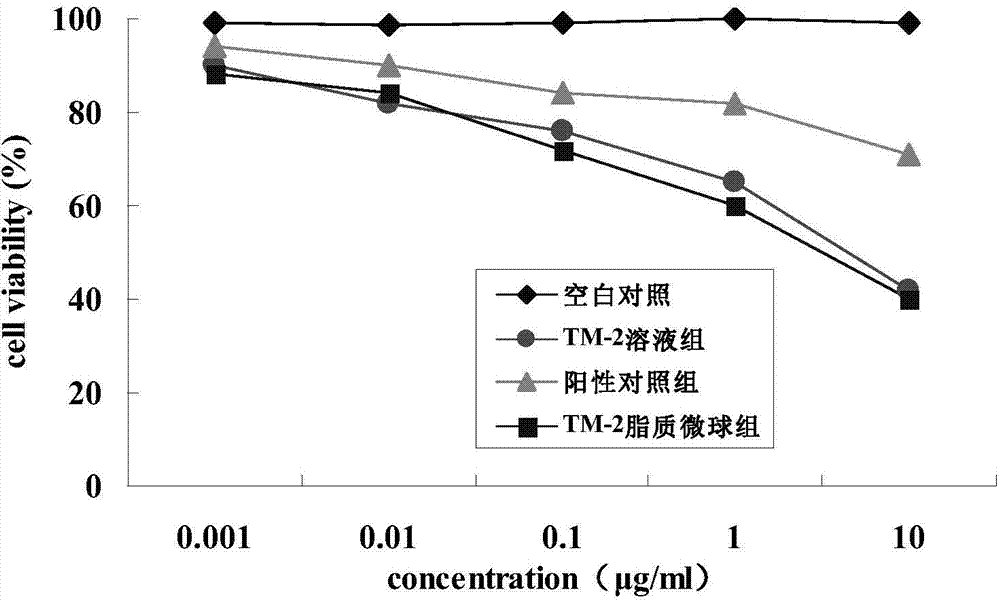
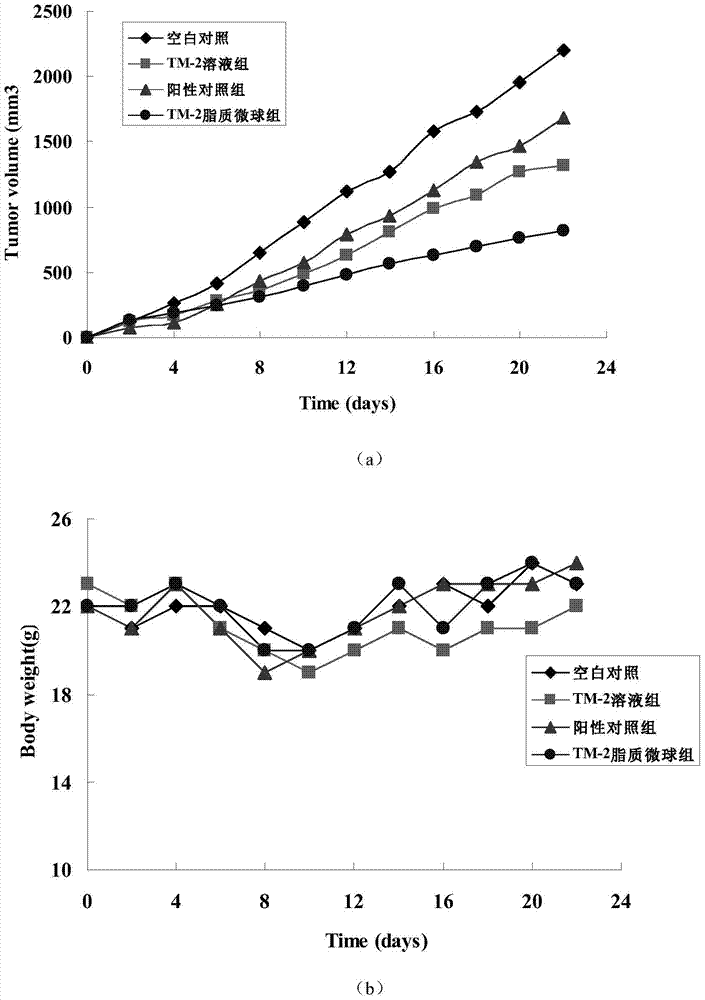
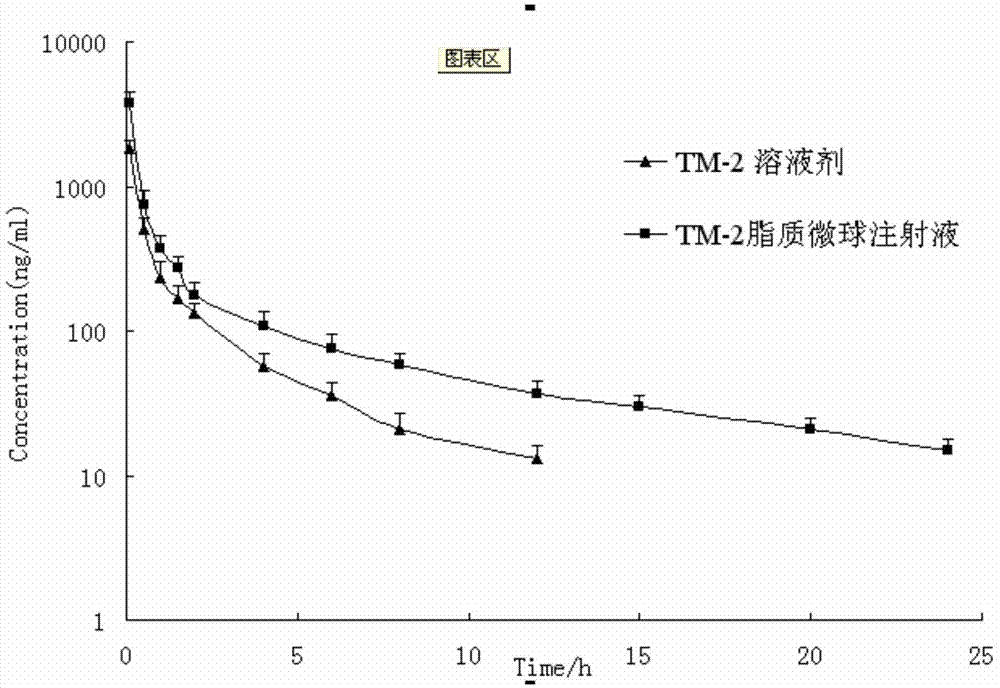
Lipid microsphere injection of taxane derivative TM-2 and preparation method thereof
A technology of TM-2 and lipid microspheres, applied in the field of medicine, can solve the problems of poor water solubility of TM-2, physical and chemical stability, increase drug encapsulation rate, prolong drug half-life, etc., and achieve increased physical and chemical stability, The effect of reducing local and vascular irritation and protecting drug activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Preparation of TM-2 lipid microsphere injection; specification: 0.01mg / ml
[0033]
[0034] Preparation:
[0035] (1) Weigh the prescription amount of glycerin, poloxamer 188 and polyethylene glycol-polyaspartic acid copolymer, add appropriate amount of water for injection, heat and stir in a magnetic stirrer at 30-80°C to disperse evenly to obtain a water phase .
[0036] (2) Weigh the prescription amount of medium-chain triglyceride (MCT), egg yolk lecithin, oleic acid, cholesterol and TM-2, stir and disperse evenly at 30-80° C. to obtain a clear drug-containing oil phase.
[0037] (3) Add the drug-loaded oil phase into the water phase under stirring by a high-speed tissue grinder, and stir at 8000 rpm for 3-5 minutes.
[0038] (4) Cool the colostrum to room temperature, dilute to full volume with water for injection, adjust the pH value to 4.0-9.0 with sodium hydroxide or hydrochloric acid solution, transfer to a high-pressure homogenizer, and control the homoge...
Embodiment 2
[0041] Preparation of TM-2 lipid microsphere injection, specification: 10mg / ml
[0042]
[0043] Preparation:
[0044] (1) Weigh the prescription amount of glycerin, poloxamer 188 and polyethylene glycol-polyaspartic acid copolymer, add appropriate amount of water for injection, heat and stir in a magnetic stirrer at 30-80°C to disperse evenly to obtain a water phase .
[0045] (2) Weigh soybean oil, medium-chain triglyceride (MCT), egg yolk lecithin, oleic acid, cholesterol and TM-2 at 30-80° C. and stir to disperse evenly to obtain a clear medicated oil phase.
[0046] (3) Add the drug-loaded oil phase into the water phase under stirring by a high-speed tissue grinder, and stir at 10,000 rpm for 3-5 minutes.
[0047] (4) Cool the colostrum to room temperature, dilute to full volume with water for injection, adjust the pH value to 4.0-9.0 with sodium hydroxide or hydrochloric acid solution, transfer to a high-pressure homogenizer, and control the homogenization temperatu...
Embodiment 3
[0050] Preparation of TM-2 Lipid Microsphere Injection Specification: 2mg / ml
[0051]
[0052] Preparation:
[0053] (1) Weigh the prescription amount of glycerin, poloxamer 188 and polyethylene glycol-polyaspartic acid copolymer, add appropriate amount of water for injection, heat and stir in a magnetic stirrer at 30-80°C to disperse evenly to obtain a water phase .
[0054] (2) Weigh soybean oil, medium-chain triglyceride (MCT), egg yolk lecithin, oleic acid, cholesterol and TM-2 at 30-80° C. and stir to disperse evenly to obtain a clear medicated oil phase.
[0055] (3) Add the drug-loaded oil phase into the water phase under stirring by a high-speed tissue grinder, and stir at 10,000 rpm for 3-5 minutes.
[0056] (4) Cool the colostrum to room temperature, dilute to full volume with water for injection, adjust the pH value to 4.0-9.0 with sodium hydroxide or hydrochloric acid solution, transfer to a high-pressure homogenizer, and control the homogenization temperature...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com