Application of diosgenin-3-site derivative
A technology of diosgenin and derivatives, which is applied in the field of diosgenin-3-position derivatives, can solve the problem of drugs for neovascular ophthalmopathy without convenient eye-drop administration, high treatment cost and large molecular weight, which cannot penetrate the fundus of the eyes Issues such as drug efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
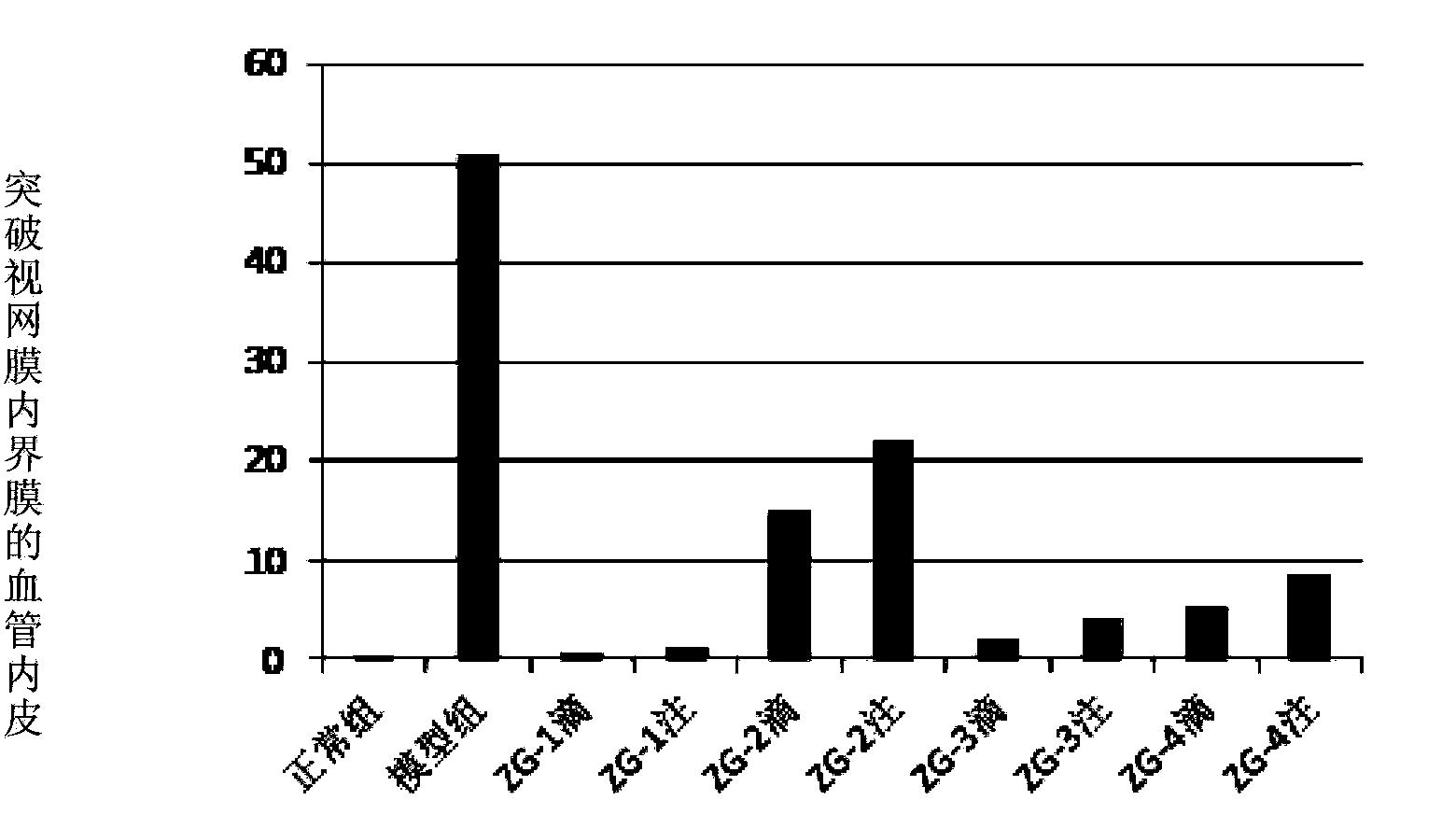
Image
Examples
Embodiment 1
[0055] Preparation of Example 1 Compound 4 (ZG-1 for short)
[0056]
[0057] a. N-tert-butoxycarbonyl-6-aminocaproic acid diosgenin ester
[0058]Diosgenin (12.5g), compound 2 (7.0g) and a catalytic amount of DMAP were dissolved in anhydrous dichloromethane, and an appropriate amount of EDC was added to react for 6 hours at room temperature. The filtrate was successively washed with dilute sodium chloride solution, saturated sodium bicarbonate solution and water, dried over anhydrous sodium sulfate, and separated on a silica gel column (petroleum ether / ethyl acetate 12:1) to obtain the white solid compound 3N-tert-butoxycarbonyl- Diosgenin 6-aminocaproate (15.2g, 80%). 1 H NMR (400MHz, CDCl3) δ5.37(d, J=4.3Hz, 1H), 4.67–4.50(m, 2H), 4.41(dd, J=15.0, 7.4Hz, 1H), 3.67(s, 1H) ,3.53–3.43(m,1H),3.37(t,J=10.9Hz,1H),3.11(d,J=6.3Hz,2H),2.38–2.21(m,4H),2.08–1.91(m,2H ), 1.04(s,3H), 0.97(d,J=6.9Hz,3H),0.79(d,J=3.5Hz,6H).
[0059] b. 6-aminocaproic acid diosgenin ester
[0060] ...
Embodiment 2
[0061] Preparation of Example 2 Compound 9 (ZG-4 for short)
[0062]
[0063] Referring to the method in Example 1, the amino acid raw materials for the reaction were changed to obtain Compound 9. 1 H NMR (400MHz,MeOD)δ5.33(d,J=5.0Hz,1H),4.67–4.49(m,1H),4.30(dd,J=14.6,7.6Hz,1H),3.85(t,J= 6.4Hz, 1H), 3.39–3.30(m, 1H), 2.93–2.76(m, 2H), 2.41–2.20(m, 2H).
Embodiment 3
[0064] The preparation method of embodiment 3 compound 14 (abbreviated as ZG-3)
[0065]
[0066] a. N-tert-butoxycarbonyl-6-aminocaprylic acid diosgenin ester
[0067] Diosgenin dios (4.14g), compound 12 (2.59g), and DMAP (244mg) were dissolved in anhydrous dichloromethane, and DCC (2.48g) was added at room temperature to react overnight, and filtered. The filtrate was successively washed with dilute sodium chloride solution, saturated sodium bicarbonate solution and water, dried over anhydrous sodium sulfate, and separated on a silica gel column (petroleum ether / ethyl acetate 10:1) to obtain the white solid compound 13N-tert-butoxycarbonyl- 8-aminocaprylic acid diosgenin ester. 1 H NMR (400MHz, CDCl 3 )δ5.30(d,J=5.0Hz,1H),4.60–4.46(m,1H),4.34(dd,J=14.9,7.5Hz,1H),3.44–3.36(m,1H),3.30(t ,J=10.9Hz,1H),3.02(t,J=7.0Hz,2H),2.30–2.14(m,4H),1.98–1.86(m,2H),0.72(t,J=3.1Hz,6H) .
[0068] b. 8-aminocaprylic acid diosgenin ester
[0069] Compound 13 (5.25 g) was dissolved in di...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com