Compound antihypertensive preparation and application of compound antihypertensive preparation
A technology of high blood pressure and receptor antagonists, which is applied in the field of pharmaceutical compositions with metolazone as the active ingredient and angiotensin Ⅱ receptor antagonists, to achieve enhanced synergistic antihypertensive effect, a wide range of drug users, and improved compliance Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
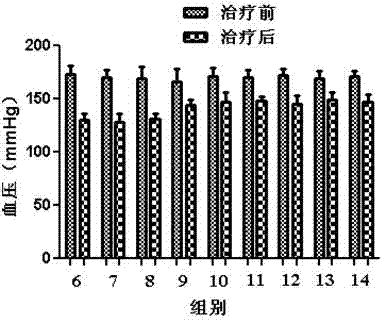
Image
Examples
Embodiment 1
[0076] Embodiment 1: Preparation of compound pharmaceutical composition 1 (tablet) (calculated in 1000 tablets)
[0077] Tablet core prescription :
[0078] Valsartan 80
[0079] Metolazone 0.5
[0080] Microcrystalline Cellulose 60
[0081] starch 20
[0082] Crospovidone 10
[0083] Croscarmellose Sodium 4.5
[0084] Micronized silica gel 3
[0086] Coating prescription
[0087] Opadry Coating Powder 4g
[0088] Purified water 45g
[0089] Preparation:
[0090] (1) Weigh the prescribed amount of valsartan and metolazone, crush them through an 80-mesh sieve, and mix them evenly by using the method of equal increments.
[0091] (2) Weigh the prescribed amount of microcrystalline cellulose, starch, crospovidone, croscarmellose sodium, and micropowder silica gel, crush them through a 60-mesh sieve, and mix well.
[0092] (3) Mix (1) and (2) evenly, then add magnesium stearate, and mix evenly to obtain an intermediate.
[0093]...
Embodiment 2
[0096] Embodiment 2: Preparation of compound pharmaceutical composition 2 (tablet) (calculated in 1000 tablets)
[0097] Tablet prescription:
[0098] Valsartan 160g
[0099] Metolazone 0.25
[0100] Microcrystalline Cellulose 80g
[0101] Starch 26.5g
[0102] Crospovidone 15g
[0103] Croscarmellose Sodium 10g
[0104] Micronized silica gel 6g
[0105] Magnesium Stearate 2.5g
[0106] Coating prescription:
[0107] Opadry Coating Powder 4g
[0108] Purified water 45g
[0109] Preparation :
[0110] (1) Weigh the prescribed amount of valsartan and metolazone, crush them through an 80-mesh sieve, and mix them evenly by using the method of equal increments.
[0111] (2) Weigh the prescribed amount of microcrystalline cellulose, starch, crospovidone, croscarmellose sodium, and micropowder silica gel, crush them through a 60-mesh sieve, and mix well.
[0112] (3) Mix (1) and (2) evenly, then add magnesium stearate, and mix evenly to obtain an intermediate.
[0...
Embodiment 3
[0116] Embodiment 3, the preparation of compound pharmaceutical composition 3 (capsules) (in 1000 grains)
[0117] prescription :
[0118] Valsartan 40g
[0119] Metolazone 1g
[0120] Microcrystalline Cellulose 50 g
[0121] Crospovidone 15g
[0122] Povidone K30 12g
[0123] Sodium Lauryl Sulfate 0.8g
[0124] Magnesium stearate 1.2g
[0125] Preparation:
[0126] (1) Raw materials and auxiliary materials are passed through 80-mesh sieve respectively, and set aside.
[0127] (2) Take povidone K30 and sodium lauryl sulfate, dissolve in warm water to 50ml, and use it as a binder;
[0128] (3) Weigh valsartan, metolazone, microcrystalline cellulose, and crospovidone, mix them uniformly in equal increments, add binders to make soft materials, and granulate with 18 mesh sieves. Dry at ℃.
[0129] (4) After the granules are dried, sieve the granules with a 20-mesh sieve, then add magnesium stearate, and mix well.
[0130] (5) Calculate the content of intermediates...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com