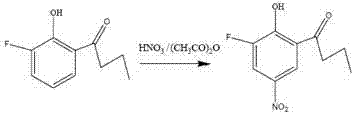
Compound 2-hydroxy-3-fluoro-5-nitro-1-phenyl butanone and preparation method and agricultural biological activity thereof
A technology of phenylbutanone and compounds, which is applied to the preparation of nitro compounds, botany equipment and methods, chemicals for biological control, etc., can solve the problems of low atom utilization rate, low mixed acid usage rate, poor selectivity, etc. , to achieve high inhibitory effect, less reaction steps and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0012] Example 1: Weigh 0.196 g (0.005629 mol) of fuming nitric acid and 0.583 g of acetic anhydride in a reaction flask, stir at 30°C for 1 h, transfer the reaction flask to an ice bath, and add in 5 batches within 1 hour 2-Hydroxy-3-fluoro-1-phenylbutanone 0.512 g. During the reaction process, the reaction progress was judged by thin-layer chromatography. After the reaction was completed, add distilled water and ethyl acetate to the reaction bottle, separate the liquids, extract twice with ethyl acetate, combine the organic phases, remove water with anhydrous sodium sulfate, and evaporate under reduced pressure to obtain a crude product, which was subjected to silica gel column chromatography. Pale yellow crystals were obtained with a yield of 67%.
Embodiment 2
[0013] Example 2: Weigh 0.196 g (0.005629 mol) of fuming nitric acid and 0.583 g of acetic anhydride in a reaction flask, stir at 30°C for 1 h, transfer the flask to a 20°C water bath, and add in 5 batches within 1 hour 2-Hydroxy-3-fluoro-1-phenylbutanone 0.512 g. During the reaction process, the reaction progress was judged by thin-layer chromatography. After the reaction was completed, add distilled water and ethyl acetate to the reaction bottle, separate the liquids, extract twice with ethyl acetate, combine the organic phases, remove water with anhydrous sodium sulfate, and evaporate under reduced pressure to obtain a crude product, which was subjected to silica gel column chromatography. Pale yellow crystals were obtained with a yield of 59%.
[0014] Characterization of the target compound 2-hydroxy-3-fluoro-5-nitro-1-phenylbutanone
[0015] Mass spectrometry analysis showed that the molecular weight of the obtained compound was 227.2, which was consistent with the the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com