A kind of preparation method of levocloperastine fendizaic acid
The technology of leverastin and cloperastine, which is applied in the field of chemical drug synthesis, can solve the problems of low total yield in the preparation process, and achieve the effects of reducing three-waste pollution, reducing costs and high safety.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
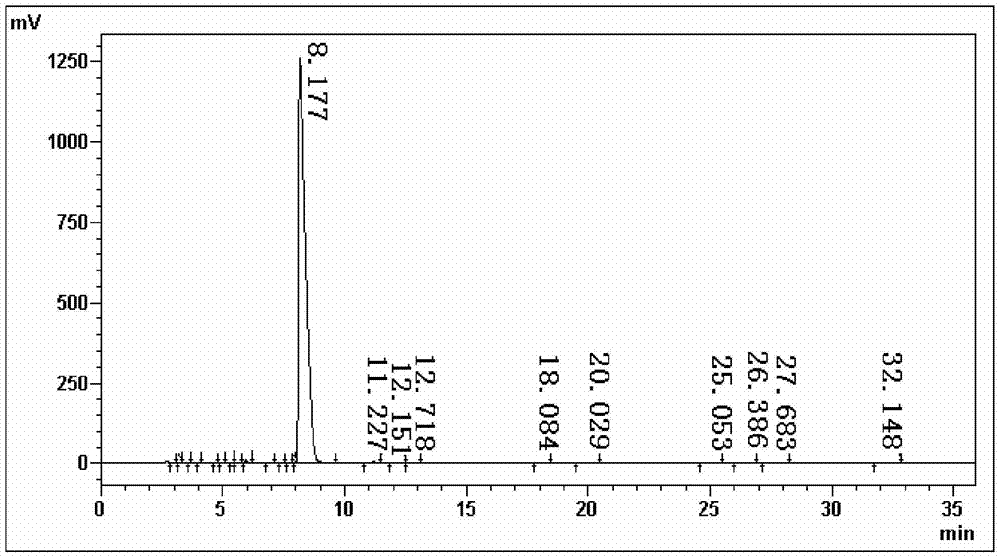
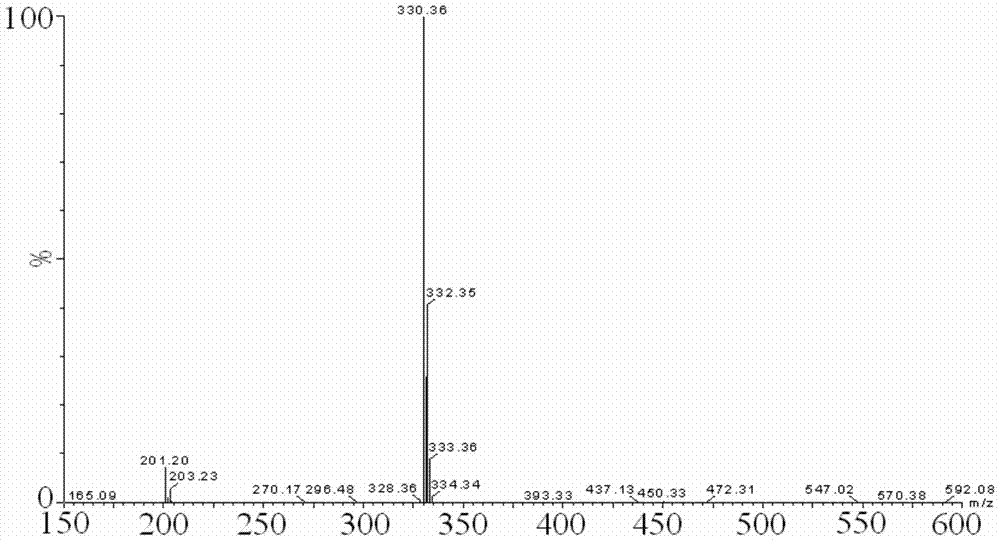
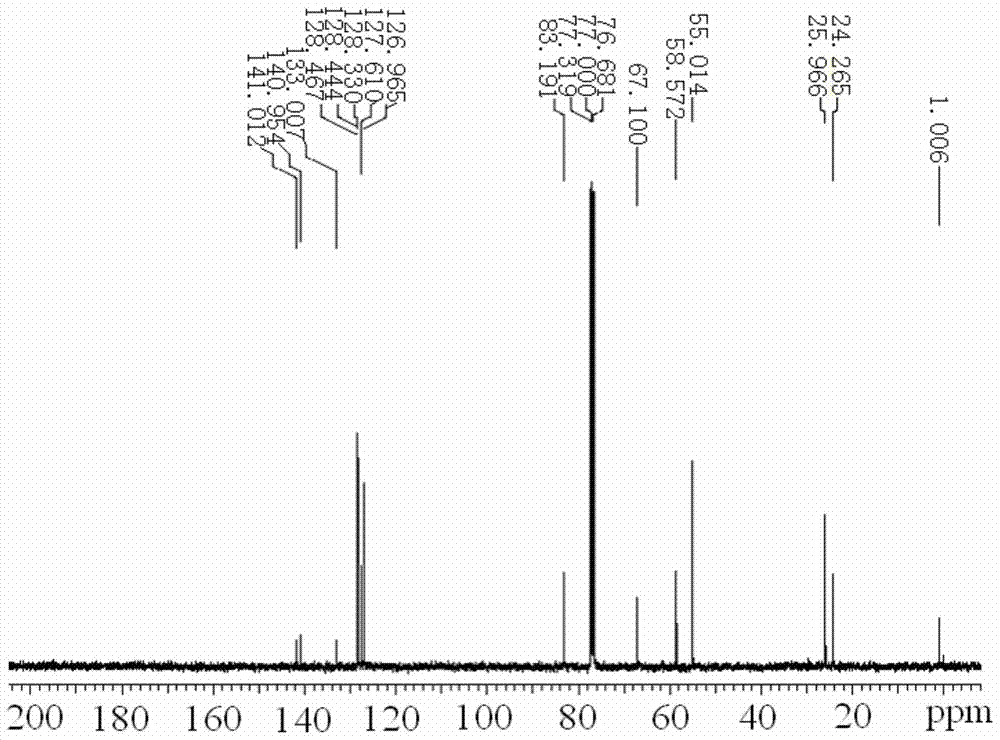
Image
Examples
preparation example Construction
[0037] The invention provides a kind of preparation method of L-cloperastine fendizac acid, comprising the following steps:
[0038] Use a resolving agent to resolve racemic cloperastine in an aliphatic alcohol solvent to obtain levocloperastine. The resolving agent is L-(-)-diR substituted benzoyl tartaric acid, and the R is alkyl, alkoxy, -Cl, -F, -Br or -H;
[0039] The levo-cloperastine is subjected to a salt-forming reaction with fendisic acid to obtain levo-cloperastine fendisic acid.
[0040] The method provided by the invention uses L-(-)-diR substituted benzoyl tartaric acid as a resolving agent to resolve racemic cloperastine in an aliphatic alcohol solvent, and the resolution yield is high, so that the obtained The levocloperastine fendioleic acid has high optical purity and high product yield.
[0041] In addition, the resolving agent and fatty alcohol solvents used in the resolution of racemic cloperastine in the present invention can be used repeatedly, which r...
Embodiment 1
[0113] Into a dry and clean 1000mL three-necked reaction flask, add 300g (1.373mol) 4-chlorobenzhydryl alcohol, 150g (1.863mol) 2-chloroethanol and 350mL toluene in sequence, and stir to dissolve;
[0114] Slowly add 50 g of concentrated sulfuric acid dropwise to the dissolved solution, heat to reflux at a heating rate of 4°C / min, and react for 5 hours;
[0115] Cool the obtained reaction product to room temperature, add 100mL of water to it for extraction, neutralize it with 42% liquid caustic soda until the pH value of the water layer reaches 7, and separate the water layer;
[0116] The organic layer was distilled under reduced pressure to recover toluene, distilled to an internal temperature of 120°C, and a vacuum of -0.08 MPa or higher to obtain 390 g of an oily substance.
[0117] Put the above 390g oil into a 2000mL dry and clean three-necked reaction flask, add 150g of anhydrous sodium carbonate and 180g (2.118mol) of piperidine to it, and heat it to 95°C at a heating ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com