Polyvalent hydroxy resin, epoxy resin, method for producing same, epoxy resin composition and cured product thereof
A polyhydroxyl resin, polyhydroxyl technology, applied in the field of epoxy resin, can solve the problem of no polyhydroxyl resin and molecular weight distribution research of epoxy resin, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0100] Hereinafter, the present invention will be described more specifically through examples. The unit of hydroxyl equivalent and epoxy equivalent is g / eq.
Synthetic example 1
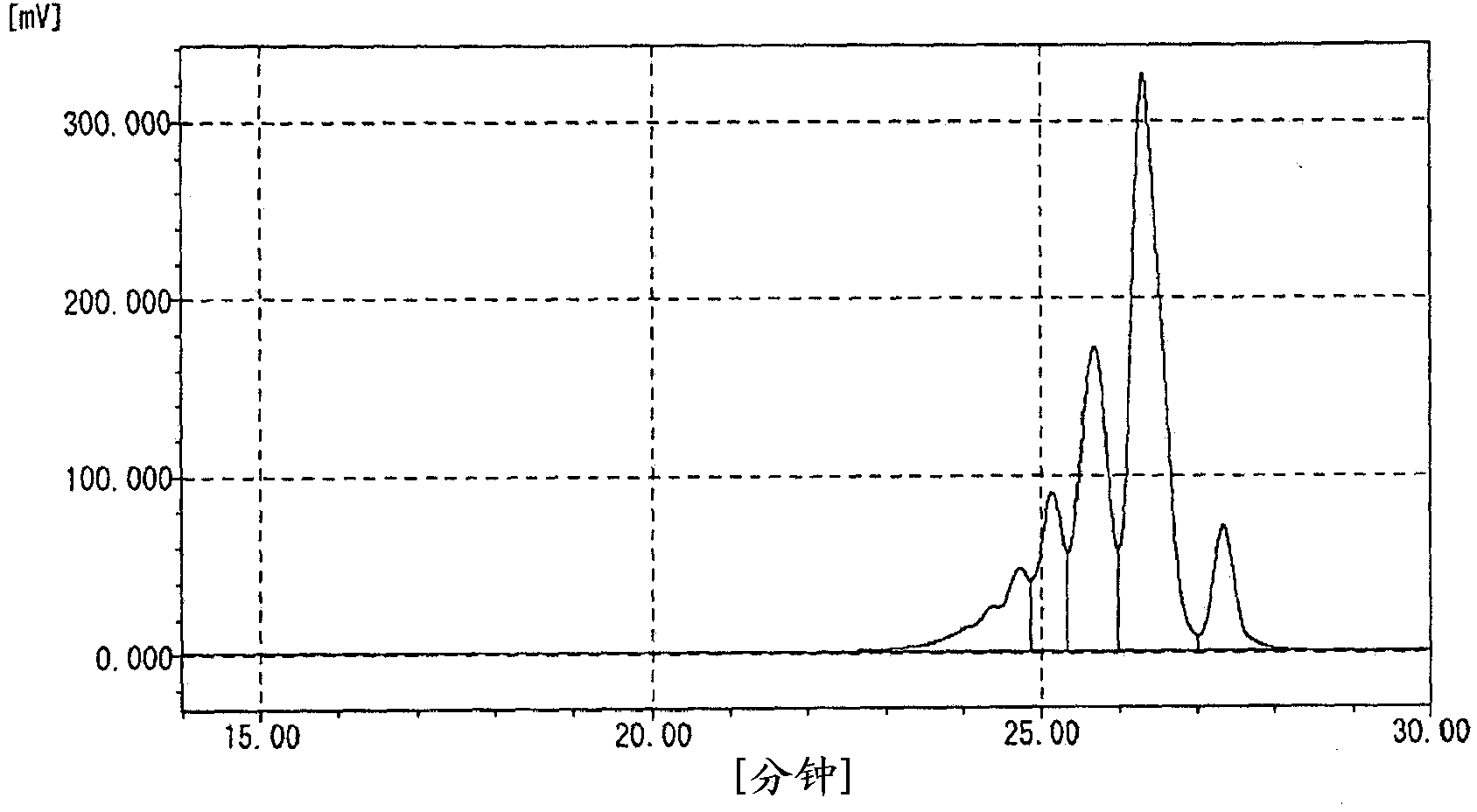
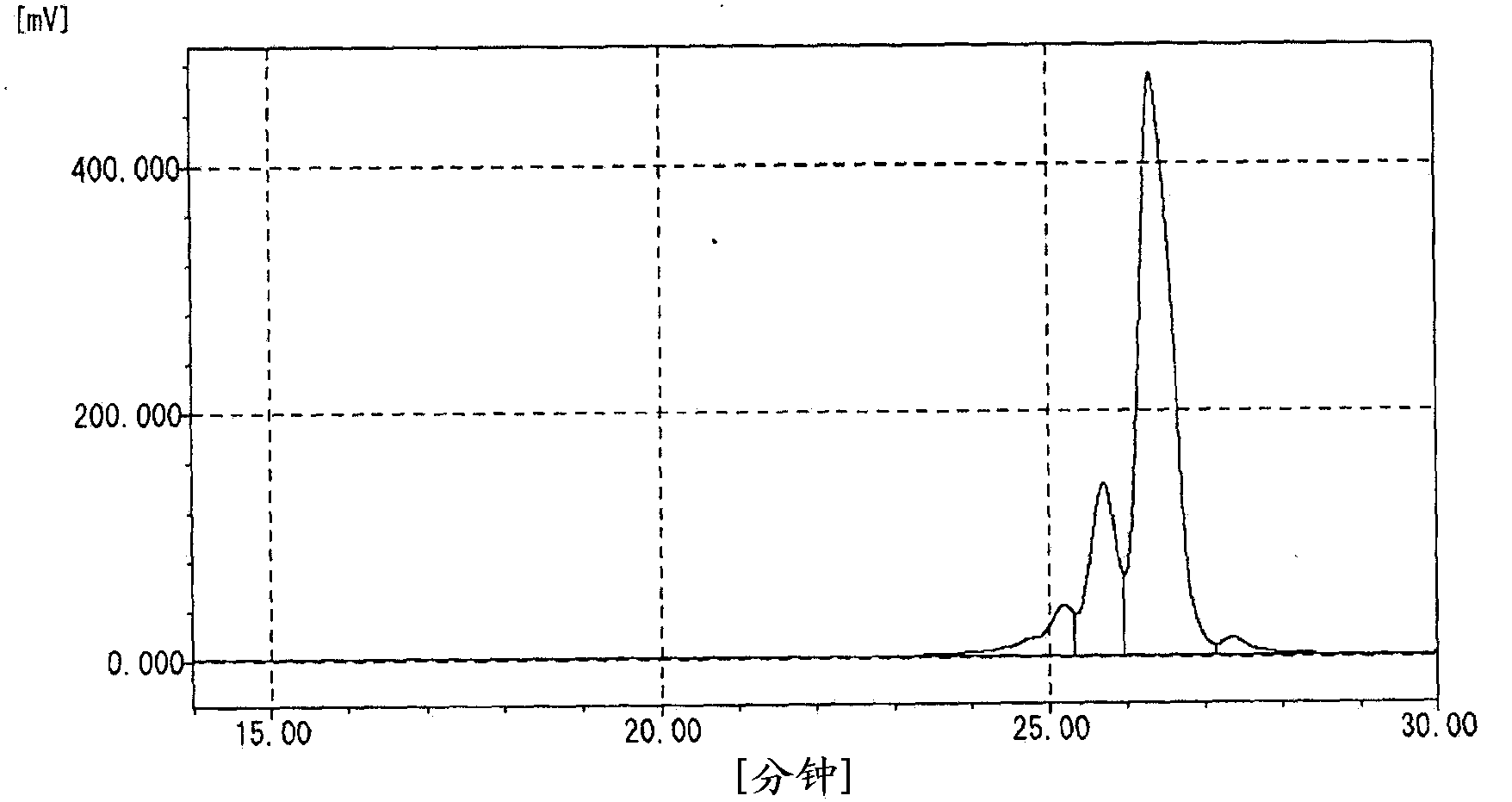
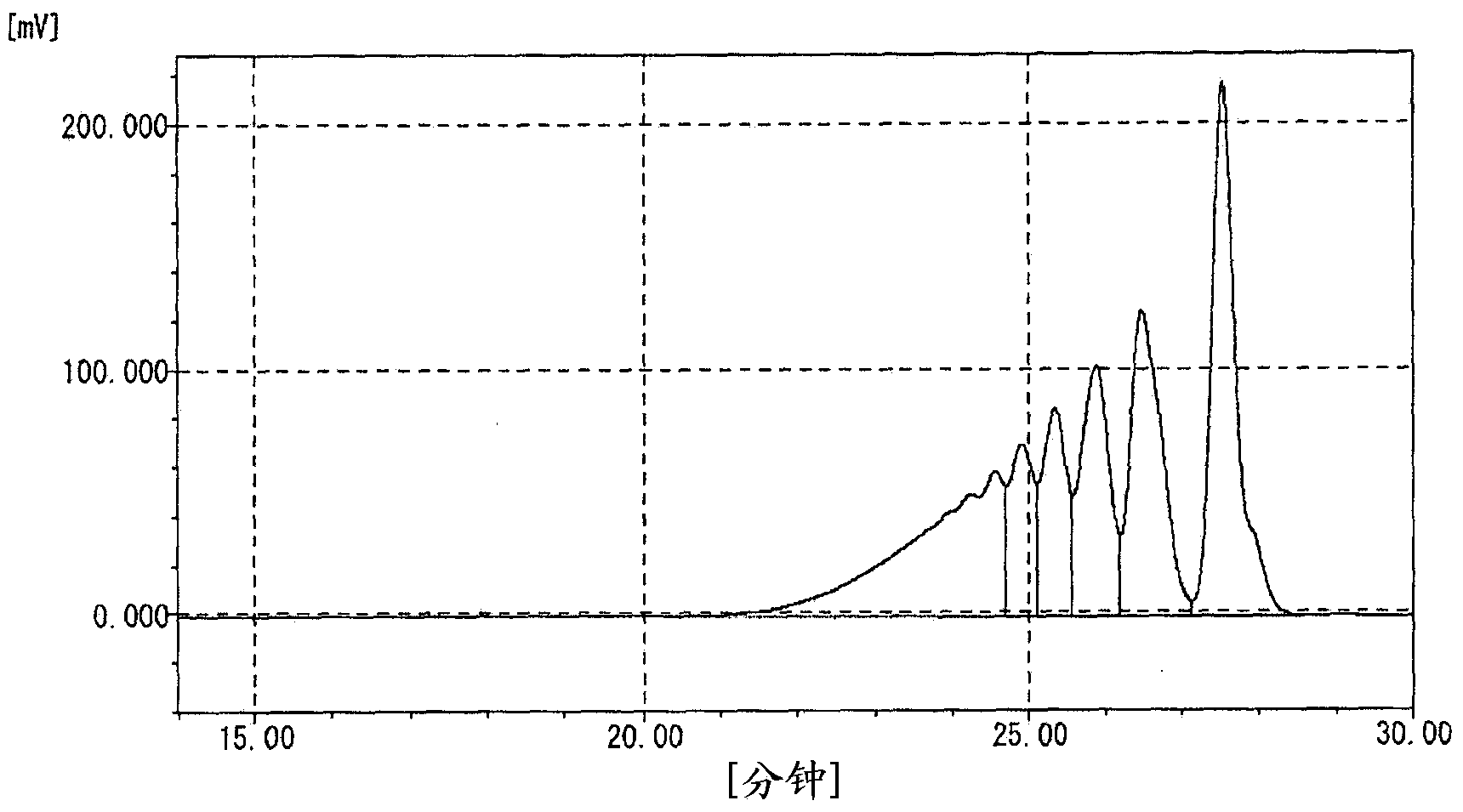
[0102] Into a 1 L four-necked flask, 250 g of phenol and 0.75 g of oxalic acid dihydrate as an acid catalyst were charged, stirred while introducing nitrogen gas, and heated to raise the temperature. Dropwise addition of 47.4 g of 37.4% formalin was started at 80° C., and the dropwise addition was completed over 30 minutes. Further, the reaction temperature was kept at 92° C., and the reaction was performed for 3 hours. The temperature was raised to 110° C. while removing the reaction product water to the outside of the system while raising the temperature. Residual phenol was recovered under reduced pressure at 160°C. A part of the dinuclear body was recovered by further raising the temperature. The hydroxyl equivalent of the obtained polyvalent hydroxy compound was 104, the softening point was 68°C, and the melt viscosity at 150°C was 0.07 Pa·s. The content rate of n=1 component measured by GPC was 8.1%, the total content rate of n=2 and n=3 components was 71.7%, and Mw / M...
Synthetic example 2
[0104] 250 g of phenol, 47.4 g of 7.4% formalin, and 150 g of 89% phosphoric acid as an acid catalyst were added to a 1 L four-necked flask, and heated under the cloudy state (two-phase mixture) formed by stirring and mixing. heat up. Further maintaining the reflux temperature, the reaction was carried out for 6 hours. Next, methyl isobutyl ketone was added to dissolve the resin portion, and then it was left still to separate into a resin solution phase and a phosphoric acid aqueous solution phase. After the phosphoric acid solution phase was removed, water washing was further performed. Next, remaining phenol was collected under reduced pressure at 160°C. A part of the dinuclear body was recovered by further raising the temperature. The hydroxyl equivalent of the obtained polyvalent hydroxy compound was 103, the softening point was 65°C, and the melt viscosity at 150°C was 0.06 Pa·s. The content rate of n=1 component measured by GPC was 2.0%, the total content rate of n=2...
PUM
| Property | Measurement | Unit |
|---|---|---|
| softening point | aaaaa | aaaaa |
| softening point | aaaaa | aaaaa |
| softening point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com