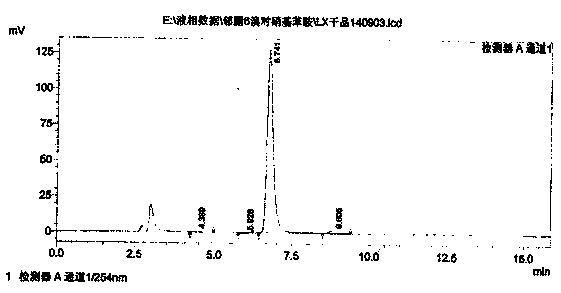
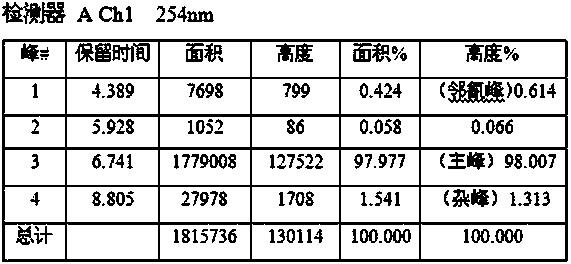
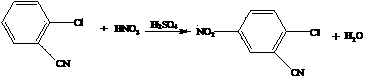Synthesis process of 2-cyano-4-nitro-6-bromaniline
A synthesis process, nitroaniline technology, applied in the synthesis process of disperse dye intermediates, 2-cyano-4-nitro-6-bromoaniline synthesis process field, can solve the problem of failure to meet quality requirements and environmental protection requirements, Unable to meet the annual output, serious pollution and other problems, to achieve the effect of low cost, reduced energy consumption, and low energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] The synthetic technique of 2-cyano-4-nitro-6-bromoaniline of the present invention comprises the following steps:
[0030] (1) Synthesis of 2-cyano-4-nitroaniline:
[0031] A1. Put 100g o-chlorobenzonitrile into 333g sulfuric acid medium, then add the mixed acid dropwise, and carry out nitration at 5-10°C to obtain the nitrated compound; the mixed acid is HNO 3 and H 2 SO 4 Mixed with a mass percentage of 0.5:1;
[0032] A2, the nitrated compound in the step A1 is subjected to water analysis, and after the water analysis, it is filtered to obtain the nitrated material;
[0033] A3, the nitrating material in step A2 is dissolved in 400g chlorobenzene, obtains nitric chloride mixed solution;
[0034] A4, carry out ammonolysis reaction with the nitric chloride mixed solution in step A3 and liquefied ammonia, distill and recover solvent at 80 ℃ after reacting to the end point; The ammonolysis reaction temperature is 110 ℃, and the ammonolysis reaction pressure is 2.6MPa...
Embodiment 2
[0045] The synthetic technique of 2-cyano-4-nitro-6-bromoaniline of the present invention comprises the following steps:
[0046] (1) Synthesis of 2-cyano-4-nitroaniline:
[0047] A1. Put 100g o-chlorobenzonitrile into 280g sulfuric acid medium, then add the mixed acid dropwise, and carry out nitration at 5-10°C to obtain the nitrated compound; the mixed acid is HNO 3 and H 2 SO 4 Mixed with a mass percentage of 1:1;
[0048] A2, the nitrated compound in the step A1 is subjected to water analysis, and after the water analysis, it is filtered to obtain the nitrated material;
[0049] A3, the nitrating material in step A2 is dissolved in 360g chlorobenzene, obtains nitric chloride mixed solution;
[0050] A4, carry out ammonolysis reaction with the nitric chloride mixed solution in step A3 and liquefied ammonia, distill and recover solvent at 100 ℃ after reacting to the end point; The ammonolysis reaction temperature is 150 ℃, and the ammonolysis reaction pressure is 4.5MPa;...
Embodiment 3
[0061] The synthetic technique of 2-cyano-4-nitro-6-bromoaniline of the present invention comprises the following steps:
[0062] (1) Synthesis of 2-cyano-4-nitroaniline:
[0063]A1. Put 100g o-chlorobenzonitrile into 300g sulfuric acid medium, then add the mixed acid dropwise, and carry out nitration at 5-10°C to obtain the nitrated compound; the mixed acid is HNO 3 and H 2 SO 4 Mixed with a mass percentage of 0.75:1;
[0064] A2, the nitrated compound in the step A1 is subjected to water analysis, and after the water analysis, it is filtered to obtain the nitrated material;
[0065] A3, the nitrating material in step A2 is dissolved in 375g chlorobenzene, obtains nitric chloride mixed solution;
[0066] A4, carry out ammonolysis reaction with the nitric chloride mixed solution in step A3 and liquefied ammonia, distill and recover solvent at 90 ℃ after reacting to the end point; The ammonolysis reaction temperature is 130 ℃, and the ammonolysis reaction pressure is 3.6MPa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com