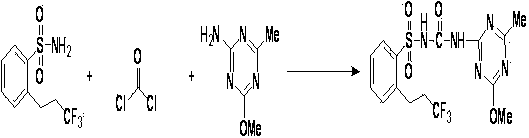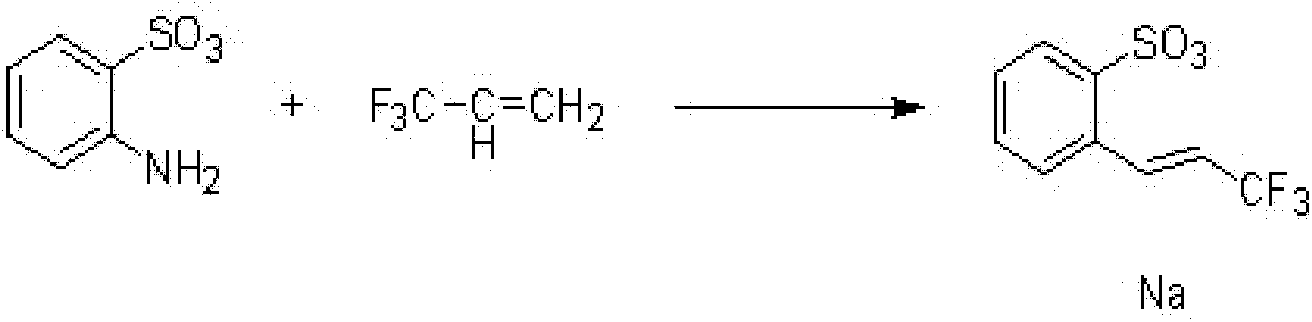Synthesis process of herbicide prosulfuron for removing gramineous weeds and broad-leaved weeds
A synthesis process, the technology of broad-leaved grass, applied in the field of synthesis process of sulfonylurea herbicide fluorsulfuron, can solve the problems of operator injury, poisoning, unfriendly compounds, etc., and achieve the effect of low toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] (1) Take a 250mL clean round-bottom flask, place it at low temperature, and control the temperature at 15°C to 20°C, add 100mL of amyl alcohol and 10mL of water, add 9.35g of anthranilic acid, 6.14g of amyl nitrite, and add acetic anhydride and 12.3g of sodium acetate, stirred at 20-30°C for 90min. Then add 1% palladium catalyst, then add 4.8g 3,3,3-trifluoro-1-propene, and continue to stir for 5h until the reaction of the raw materials is complete, and the resulting product is hydrogenated.
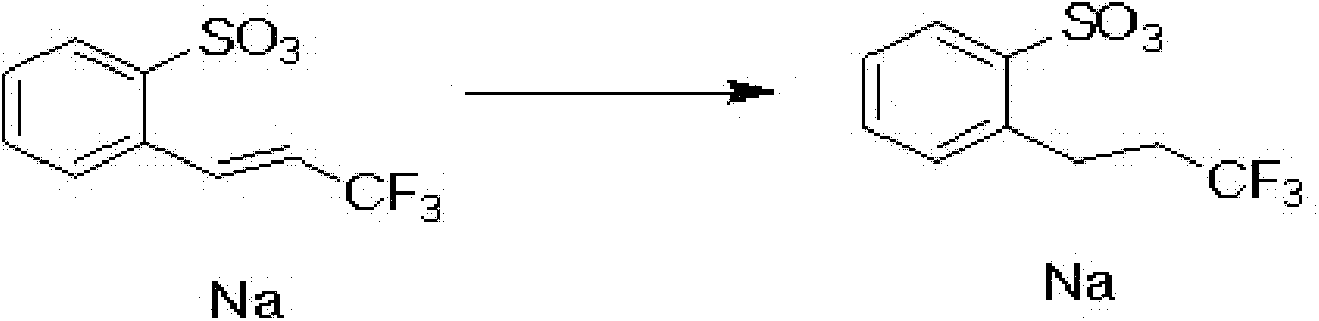
[0024] (2) Take a 50mL Schlenk tube and place it in an oil bath at 35°C-40°C, add the solution of the first step, add 0.6g carbon, control the pressure inside the Schlenk tube to 1-2 bar, and let hydrogen flow in for 6 hours After the reaction, the suspended matter was filtered out. In order to remove sodium acetate and by-products, the pentanol solution was washed with water and NaOH, the pentanol was partially distilled, and water was added to make the pentanol be azeotrop...
Embodiment 2
[0028] (1) Take a 500mL clean round-bottomed flask, place it at low temperature, and control the temperature at 15°C to 20°C, add 200mL of amyl alcohol and 20mL of water, add 18.7g of anthranilic acid, 12.28g of amyl nitrite, and add acetic anhydride and 24.6g of sodium acetate, stirred at 20-30°C for 90min. Then add 1% palladium catalyst, then add 9.6g 3,3,3-trifluoro-1-propene, and continue to stir for 5h until the reaction of the raw materials is complete, and the resulting product is hydrogenated.
[0029] (2) Take a 100mL Schlenk tube and place it in an oil bath at 35°C-40°C, add the solution of the first step, add 1.2g of carbon, control the pressure inside the Schlenk tube to 1-2 bar, and let hydrogen flow in for 6 hours After the reaction, the suspended matter was filtered out. In order to remove sodium acetate and by-products, the pentanol solution was washed with water and NaOH, the pentanol was partially distilled, and water was added to make the pentanol be az...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com