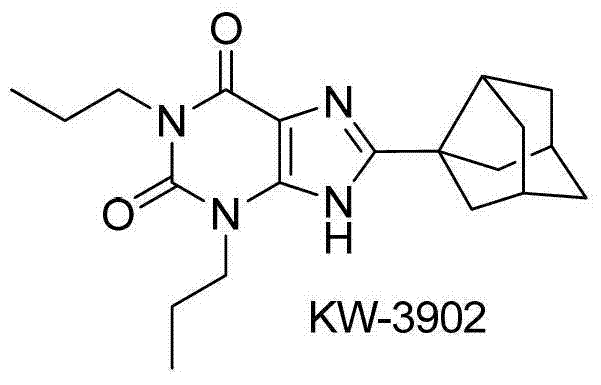
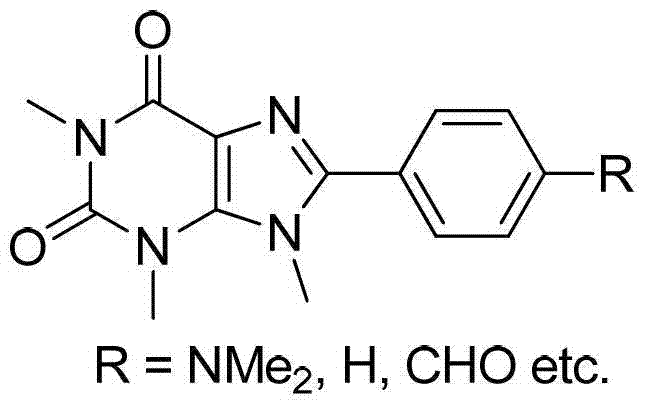
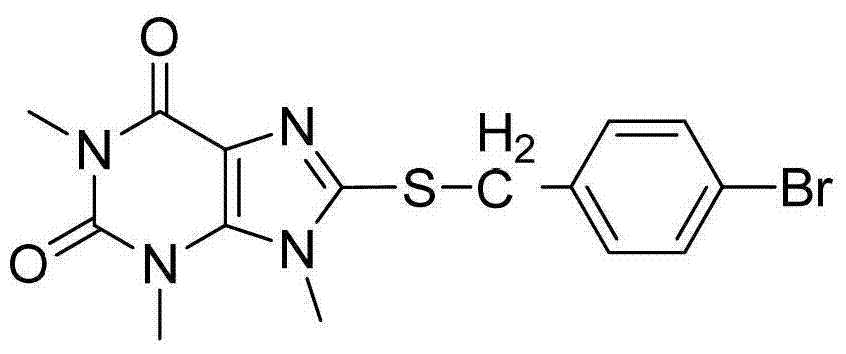
Preparation method of 8-thiaxanthine compound
A technology for disulfide compounds and xanthine, applied in the field of preparation of 8-thiaxanthine compounds, can solve the problems of low reaction atom economy, unfavorable large-scale production, difficult large-scale synthesis and the like, and achieves improved atom utilization, High productivity and waste reduction effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Add silver acetate (7mg, 0.05mmol) and copper acetate (100mg, 0.5mmol) to a 25mL round bottom flask, add 2mL DMF (N,N-dimethylformamide) to dissolve, then add caffeine 1a (385mg, 2.5mmol, that is, in formula II, R 1 , R 2 , R 3 Both are methyl) and diphenyl disulfide 2a (325mg, 1.5mmol, that is, in formula III, R 4 for phenyl) to form a reaction system. After 12 hours at 120°C, the reaction system was quenched by adding 5 mL of water, and extracted three times with dichloromethane (10 mL). After the organic phases were combined and washed with saturated brine, the organic layer obtained was dried over anhydrous sodium sulfate, and the solvent was removed by rotary evaporation, and then separated by silica gel (300-400 mesh) column chromatography (eluent: dichloromethane and acetone) Volume ratio = 20:1) to obtain 717.5 mg of 1,3,7-trimethyl-8-phenylthioxanthine (yield: 95%) as a white solid. The melting point of the product is 135-137°C; 1 H NMR (CDCl 3 ,600MHz):...
Embodiment 2
[0059] Except that the copper acetate of 2.5mmol is used to replace the copper acetate of 0.5mmol in Example 1 as the catalyst, the remaining operating steps are the same as in Example 1 to obtain 1,3,7-trimethyl-8-phenylthioxanthine 3aa, producing Yield 85%, product characterization data are with embodiment 1.
Embodiment 3
[0061] Except that the silver acetate of 0.125mmol is used to replace the silver acetate of 0.05mmol in Example 1 as an additive, the remaining operation steps are the same as in Example 1 to obtain 1,3,7-trimethyl-8-phenylthioxanthine 3aa, producing Yield 91%, product characterization data with embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com