Preparation method and application of cangrelor intermediate
A synthetic method and reaction technology, applied in the field of preparation of cangrelor intermediates
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
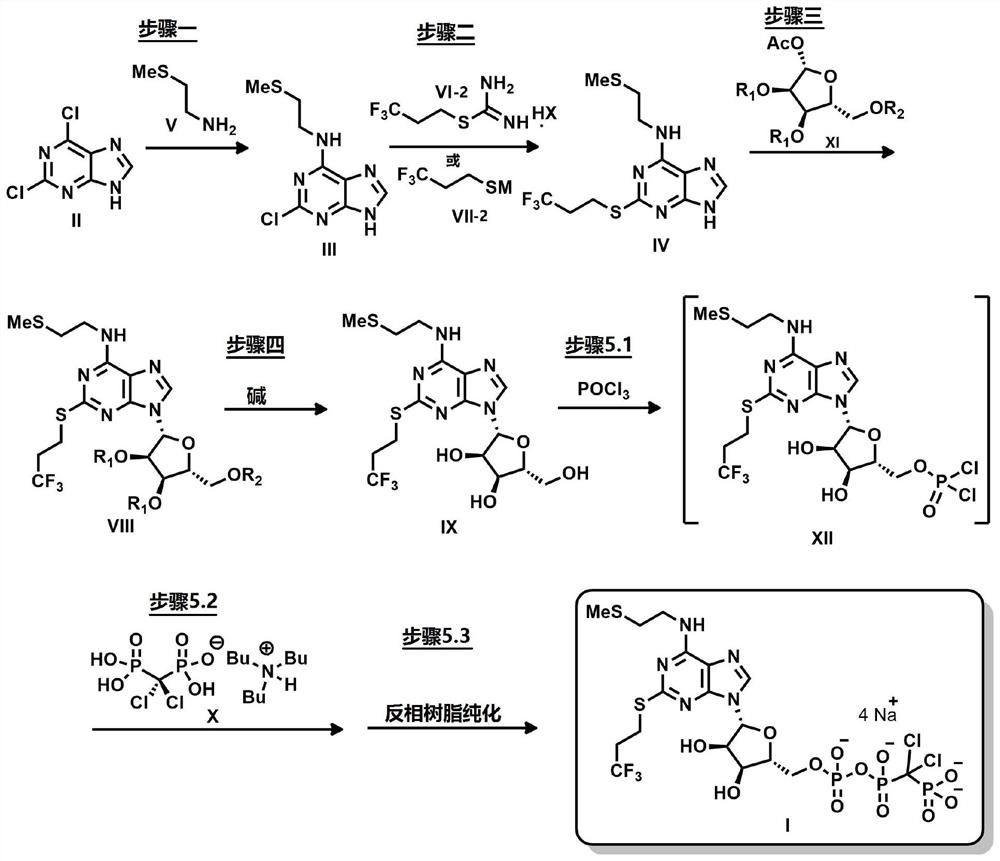
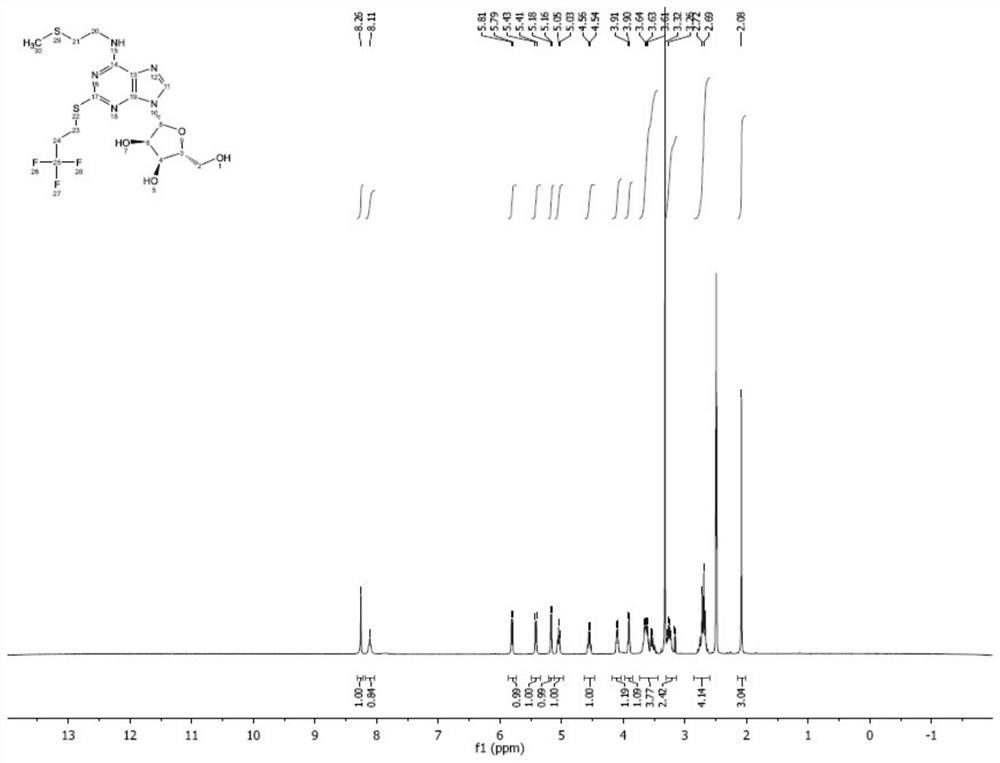
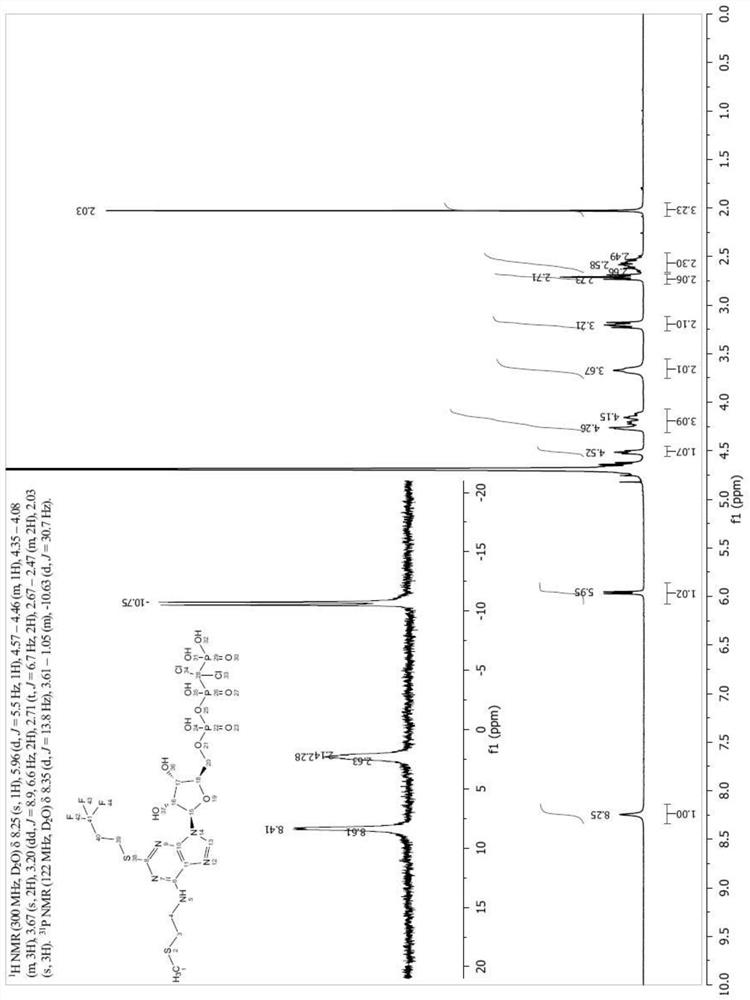
Image
Examples
Embodiment 1
[0082] Example 1: 2-chloro-N-(2-(methylthio)ethyl)-7H-purin-6-amine (2-chloro-N-(2-(methylthio)ethyl)-7H-purin-6 -amine, for the synthesis of the compound shown in formula III)
[0083] In a 100 ml flask, add 2-(methylthio)ethylamine (6.8 g, 57.5 mmol, 1.2 eq), butanol (50 ml), triethylamine (11.6 g, 115 mmol, 2 eq) and 2,6-Dichloropurine (8.96 g, 48 mmol, 1 equiv). The mixture was heated to 100°C and stirred for 6 hours. The mixture was cooled to room temperature and filtered to obtain a white filter cake. The filter cake was added to 150 ml of saturated sodium bicarbonate solution and stirred for 30 minutes, filtered, and the filter cake was washed with water (100 ml). The filter cake was dried in vacuo to obtain 10.9 g of the product, the compound of formula III. Yield 93%, HPLC purity 96%.
[0084] Proton spectrum data of the compound shown as formula III: 1 H NMR (300MHz,D 6 -DMSO)δ12.93(s,1H),8.17(s,1H),8.12(s,1H),3.62(dd,J=13.4,6.4Hz,2H),2.7(q,2H),2.1(s ,3H).
Embodiment 2
[0085] Example 2: Synthesis of 2-chloro-N-(2-(methylthio)ethyl)-7H-purin-6-amine (compound as shown in formula III)
[0086]In a 250 ml flask, add 2-(methylthio)ethylamine (21 g, 230 mmol, 1.5 eq), ethanol (50 ml), triethylamine (39 g, 389 mmol, 2.5 eq) and 2 , 6-Dichloropurine (29 g, 150 mmol, 1 equiv). The mixture was heated to 100°C and stirred for 6 hours. The mixture was cooled to room temperature, 200 ml of water were added and stirring was continued for 30 minutes. Filter to obtain a white filter cake. The filter cake was added to 400 ml of saturated sodium bicarbonate solution and stirred for 30 minutes, filtered, and the filter cake was washed with water (150 ml). The filter cake was dried in vacuo to yield 28.4 g of product, the compound of formula III. Yield 78%, HPLC purity 97%.
Embodiment 3
[0087] Example 3: 6-N-(2-(methylthio)ethyl)-2-((3,3,3-trifluoropropyl)thio)-9H-purine (compound shown in formula IV )Synthesis
[0088] Compound (0.243 gram, 1 equivalent, 1 mmol) as shown in formula III, isothiourea salt compound (compound as shown in formula VI-2, 0.45 gram, 1.5 equivalent, 1.5 mmol) and sodium hydroxide ( 120 mg, 3 equivalents, 3 mmol) were dissolved in 2 ml of dry DMF, heated to 100°C and stirred for 3 days. If the reaction is not complete, add an isothiouronium salt compound (compound as shown in formula VI-2, 0.3 g, 1 equivalent, 1 mmol) and NaOH (0.08 mg, 2 equivalents, 2 mmol) and continue heating to 115 ° C React for 24 hours. The progress of the reaction was monitored by HPLC, the reaction was cooled to room temperature and water (6 mL) was added. The mixture was stirred for 30 minutes and a pale yellow precipitate formed. The solid was isolated by vacuum filtration and the filtrate was discarded. The filter cake was washed with water (20ml). D...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com