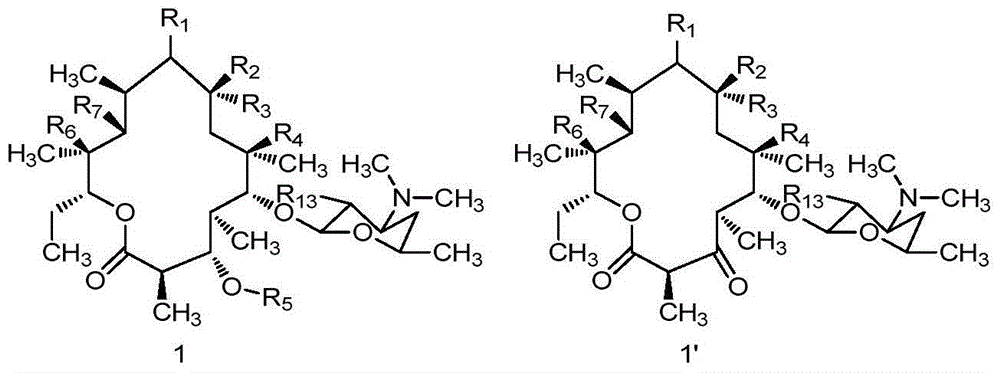
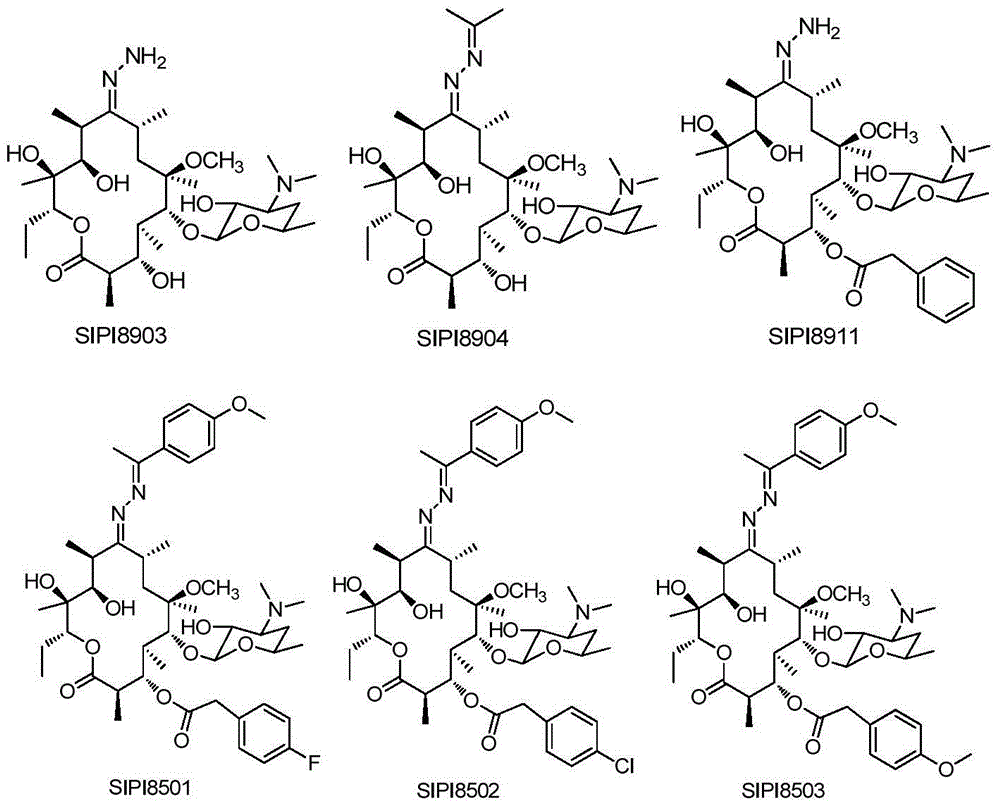
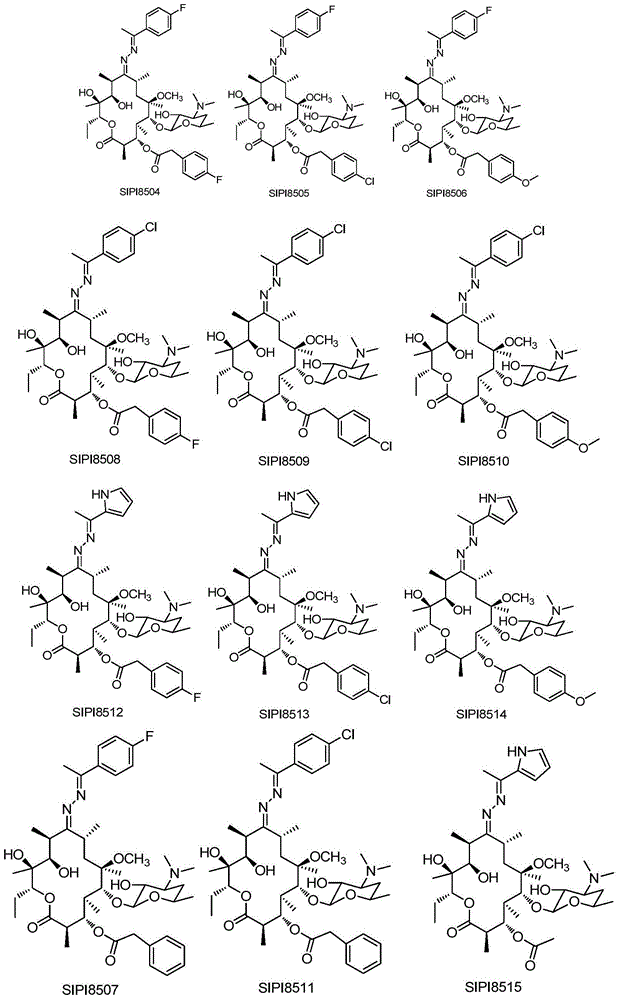
Macrolactone compounds or salts of macrolactone compounds, as well as synthesis method, pharmaceutical composition and application of macrolactone compound or salts
A technology of macrolides and compounds, applied in macrolide compounds or their salts, synthesis, pharmaceutical compositions and their application fields, and can solve problems such as defects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0259] Example 1 Preparation of 9-hydrazone clarithromycin (SIPI8901)
[0260] Clarithromycin (10g, 13.37mmol) was dissolved in methanol (80mL), hydrazine acetate (36.9g, 0.4mol) was added, and heated at reflux at 70°C for 48h. After removing part of the methanol by rotary evaporation, water (200 mL) was added, the pH was adjusted to 9-10 with 3N NaOH aqueous solution, filtered, the filter cake was washed with water, and dried to obtain 10.7 g of crude white solid. 1 g of the crude product was separated by FLASH column chromatography to obtain 0.68 g of the product (yield 72.8%, HPLC purity 85%).
Embodiment 2
[0261] Example 2 Preparation of 3-desclardinose-3-hydroxyl-9-hydrazone clarithromycin (SIPI8903)
[0262] 9-hydrazone clarithromycin (1.5g, 2mmol) was dissolved in 10mL of 1N hydrochloric acid aqueous solution, stirred at 25°C for 4h. Add 10mL of dichloromethane, adjust the pH to 9-10 with 3N sodium hydroxide, separate the layers, extract the water layer with 5mL of dichloromethane, combine the dichloromethane layers, wash with 10mL of water, dry with saturated sodium chloride, add silica gel to mix the sample Afterwards, 0.85 g of the product was obtained by separation by FLASH column chromatography. (Yield 71.4%, HPLC purity 85%).
Embodiment 3
[0263] Example 3 Preparation of 3-desclardinose-3-hydroxyl-9-isopropylidene hydrazone clarithromycin (SIPI8904)
[0264] 3-desclardinose-3-hydroxy-9-hydrazone clarithromycin (1.2g, 2mmol) was dissolved in 5mL of acetone, heated to reflux at 56°C for 4h, evaporated to dryness to obtain a crude product, mixed with silica gel, followed by FLASH column chromatography The product was isolated 0.5 g. (Yield 42.4%, HPLC purity 89.6%).
[0265] MS (ESI + ,m / e):644.85[M+H] +
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com