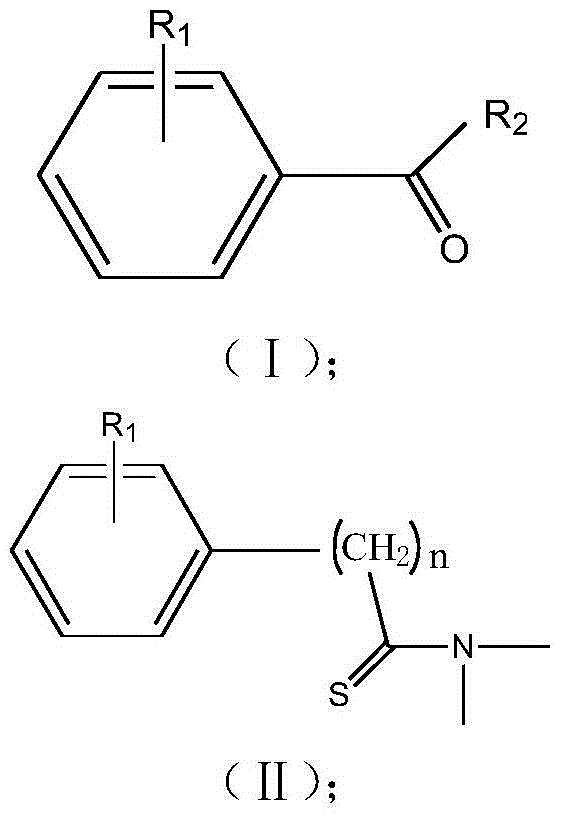
Method for preparing thioamide derivative
A technology for thioamides and derivatives, which is applied in the field of preparing thioamide derivatives, can solve the problems of low yield, limited application of thioamide derivatives, complicated product purification and processing, etc., and achieves high yield and easy operation. , the effect of fewer steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
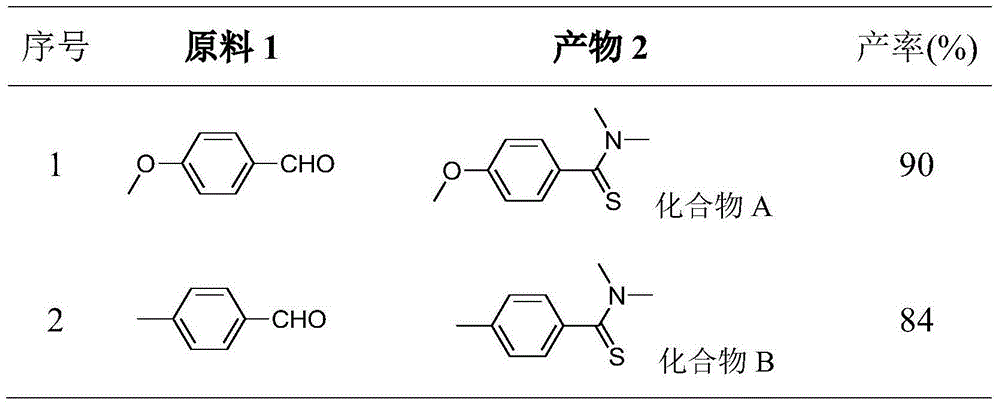
[0029] Embodiment 1: the synthesis of compound A;
[0030] Add 1.0mmol of 4-methoxybenzaldehyde, 1.2mmol of elemental sulfur, 0.2mmol of DBU and 2.0mL of DMF to the reaction vessel in sequence, and stir the resulting solution in an oil bath at 120°C for 4h, stop heating, and wait for the reaction temperature to drop to After room temperature, add silica gel to the reactant, spin off the solvent after being adsorbed for a certain period of time, and elute and separate the silica gel with the product adsorbed by thin-layer chromatography according to the volume ratio of petroleum ether: ethyl acetate = 10:1 to obtain yellow Crystalline product A, the structure of the compound was confirmed by comparison with known compounds by NMR.
Embodiment 2
[0031] Embodiment 2: the synthesis of compound B;
[0032] Add 1.0mmol of 4-methylbenzaldehyde, 1.2mmol of elemental sulfur, 0.1mmol of DBU and 3.0mL of DMF to the reaction vessel in sequence, and stir the resulting solution in an oil bath at 120°C for 4h, stop heating, and wait for the reaction temperature to drop to room temperature Finally, add silica gel to the reactant, spin off the solvent after being adsorbed for a certain period of time, and elute and separate the silica gel with the product adsorbed by thin layer chromatography according to the volume ratio of petroleum ether: ethyl acetate = 10:1 to obtain yellow crystals Product B, the structure of the compound was confirmed by comparison with known compounds by NMR.
Embodiment 3
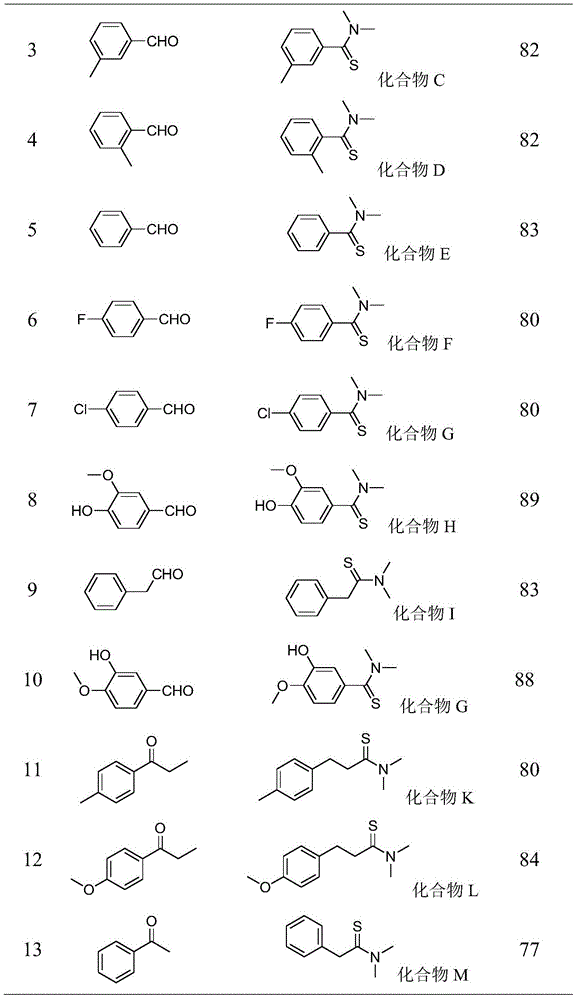
[0033] Embodiment 3: the synthesis of compound C;
[0034] Add 1.0mmol of 3-methylbenzaldehyde, 1.3mmol of elemental sulfur, 0.2mmol of DBU and 1.0mL of DMF into the reaction vessel in sequence, and stir the resulting solution in an oil bath at 100°C for 5h, stop heating, and wait for the reaction temperature to drop to room temperature Finally, add silica gel to the reactant, spin off the solvent after being adsorbed for a certain period of time, and elute and separate the silica gel with the product adsorbed by thin layer chromatography according to the volume ratio of petroleum ether: ethyl acetate = 10:1 to obtain yellow crystals Product C, the structure of the compound was confirmed by comparison with known compounds by NMR.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com