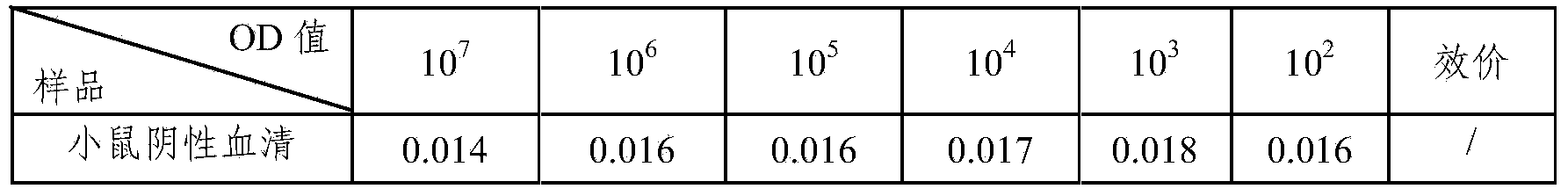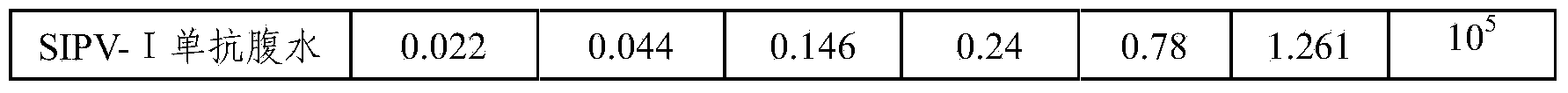Sabin strain poliomyelitis type I virus monoclonal antibody and application thereof
A poliomyelitis and monoclonal antibody technology, applied in the field of immunology, can solve the problem that the test results cannot truly reflect the content of type I antigens and the immunogenicity, etc., and achieve the effect of good virus specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1 Immunogen preparation and animal immunization
[0031] (1) Take the Vero cell working cell bank and culture it at 36.5±0.5°C after recovery until the cell concentration is 0.1-10×10 6 cells / ml, the virus was inoculated.
[0032] (2) The working seeds prepared from Intravacc-derived sabin polio type I virus were inoculated into Vero cells at MOI=10-0.05, and cultured at 32.5±0.5°C.
[0033](3) The virus was cultured for 2-4 days, and the cell supernatant was harvested, which was the poliovirus Sabin strain type I virus harvest liquid.
[0034] (4) Type I harvest liquid is clarified and concentrated more than 5 times with ultrafiltration membrane bag.
[0035] (5) Then carry out molecular sieve chromatography and ion exchange chromatography, the monitoring wavelength is 280nm, collect the eluate and flow-through respectively to obtain the purified solution, and obtain the type I vaccine stock solution after formaldehyde inactivation.
[0036] (6) Mix and emul...
Embodiment 2
[0038] Example 2 Cell fusion and strain establishment
[0039] (1) Recover and culture the SP2 / 0 cell line before cell fusion, expand the culture 3 days before fusion, remove RPMI 1640 cell culture medium (Gibco) 1 day before fusion, and add culture medium again to prepare SP2 / 0 cells.
[0040] (2) The immunized mice were sacrificed, and the mouse splenocyte suspension was prepared according to conventional methods.
[0041] (3) Add an appropriate amount of incomplete IMDM culture medium (Gibco) according to the counting results of splenocytes and SP2 / 0 cells, shake and mix the SP2 / 0 cells, and pipette the splenocytes evenly. Then the splenocytes and SP2 / 0 cells were mixed in a 50ml centrifuge tube at a ratio of 1:2 to 10:1, and mixed well.
[0042] (4) Add incomplete IMDM culture medium to 50ml, centrifuge for 5-10 minutes, and pour out the supernatant. Lightly tap the bottom of the fusion tube to loosen and evenly precipitate the cells, and place the centrifuge tube in a 3...
Embodiment 3
[0050] Example 3 Preparation of monoclonal antibody cell line ascites and detection of antibody titer
[0051] Resuscitate the frozen hybridoma cells obtained in Example 2 according to conventional methods, and cultivate them. When the cells cover more than 50% of the bottom of the 25ml cell culture bottle, BALB / c mice can be inoculated intraperitoneally according to conventional methods, and the ascites can be collected regularly. SIPV-I.
[0052] Dilute the stock solution of polio type Ⅰ vaccine with 0.01M PBS 1:20, coat 100 μl / well on a microtiter plate, overnight at 2-8°C, and detect the antibody of polio type Ⅰ ascites SIPV-I secreted by the hybridoma cells of the present invention Titer, antibody titer up to 105, higher titer. The results are shown in Table 1.
[0053] Table 1 Antibody titer test results
[0054]
[0055]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com