Application of peptidylarginine deiminase 1 (PAD1) to preparation of reagent for clinical diagnosis of tumors
A technology of peptidyl arginine deiminase and diagnostic reagent, which is applied in the application field of peptidyl arginine deiminase 1 in the preparation of tumor clinical diagnostic reagents, and can solve the problems of distribution in different tissues and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 PAD1 test example 1
[0054] PAD1 was used as a tumor blood marker to detect the blood of tumor patients. The number of tumor patients and the types of tumors are shown in Table 1.
[0055] The detection method is:
[0056] (1) Dilute the blood sample to be tested 10 times with carbonate buffer (pH 9.6), add it to a blank microtiter plate, and incubate at 37°C for 2 hours;
[0057] (2) discard the liquid in the hole, and wash the plate three times with PBST washing solution (the PBST washing solution refers to the PBS solution plus Tween-20, the pH of the PBS solution is 7.4, and the concentration of Tween-20 is 0.1%, percent by volume) ;
[0058] (3) Add 200 μL / well of blocking solution (0.5% BSA, mass percent) to each well, and incubate at 37°C for 1 hour;
[0059] (4) Discard the liquid in the well, and wash the plate three times with PBST washing solution;
[0060] (5) Add 100 μL / well of PAD1 antibody dilution (1:2000 dilution) to each group, and incubate at 37°C for 1 hour;
[0061] (6) Di...
Embodiment 2 PAD1 test example 2
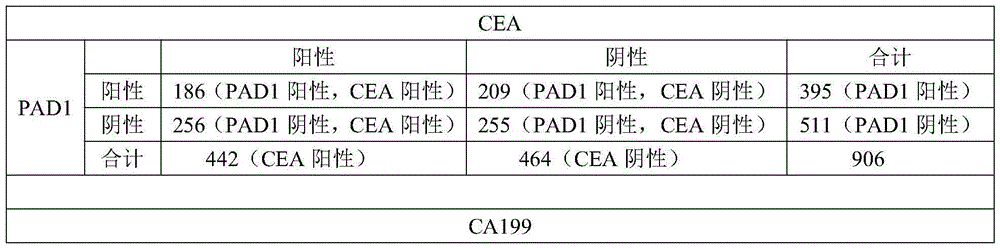
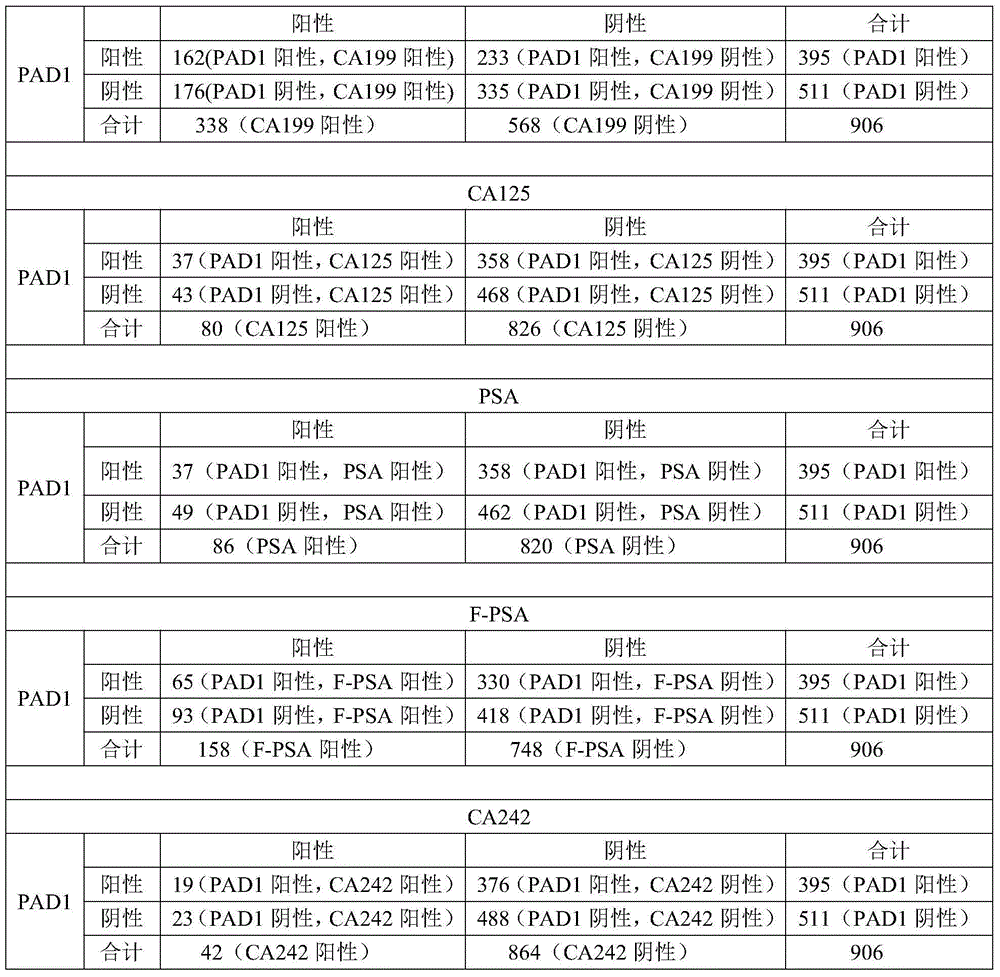
[0074] Using PAD1 as a tumor blood marker, the patient's blood was detected, and the detection method was the same as in Example 1. At the same time, with CEA, CA199, F / PSA, CA125, PSA, CA242, AFP, HCG, NSE in the prior art as tumor blood markers, the blood of tumor patients is detected (the detection method is a conventional method in the prior art). Method; the blood sample detection data of these tumor blood markers in the prior art is the patient outpatient data of a certain hospital, and this data can be used as one of the basis for diagnosing whether a patient has a tumor; the present invention uses the blood samples of some patients to detect PAD1 ). The positive rate of each tumor blood marker was compared, and the cross-comparison between PAD1 and each tumor marker.
[0075] Results: The number of patients (a total of 906 cases) and the number of positive cases are shown in Table 3. It can be seen from Table 3 that PAD1 can be used as a tumor blood marker with good ...
Embodiment 3 PAD1 test example 3
[0080] Using PAD1 as a blood marker, it is effective for hepatitis B, general inflammation (patients with increased white blood cells, neutrophils, and decreased lymphocyte ratio), kidney disease (including nephrotic syndrome, renal failure, nephritis), uremia (including uremia and urinary Road infection) patients' blood was tested, the number of patients and the type of disease are shown in Table 4.
[0081] The detection method is the same as in Example 1.
[0082] Result: The number of positive cases and the positive rate are shown in Table 4. As can be seen from Table 4, PAD1, as a blood marker, can also be used to detect hepatitis B, common inflammation (with white blood cells, neutrophils, symptoms of decreased lymphocyte ratio) ), kidney disease (including nephrotic syndrome, renal failure, nephritis), uremia (including uremia and urinary tract infection) and other diseases, the test results can be used as one of the basis for clinical diagnosis.
[0083] Table 4
[0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com