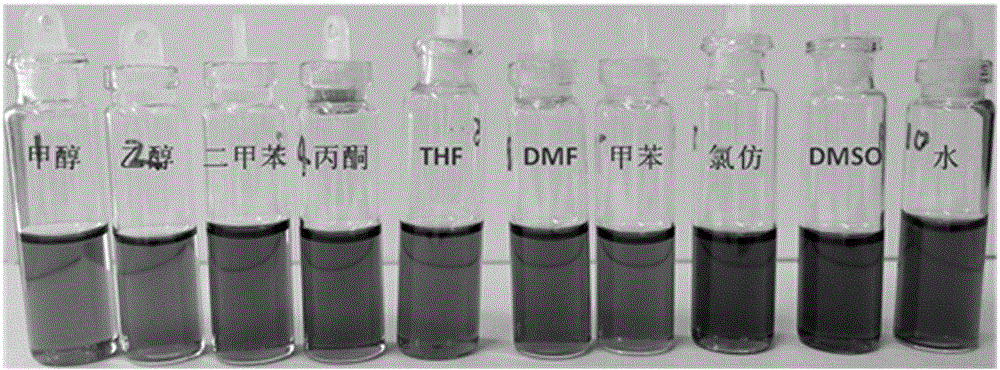
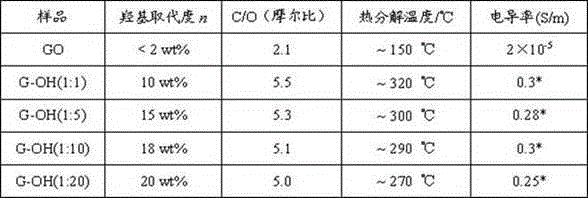
Preparation method of controllable high-substitution hydroxyl functionalized graphene
A technology with high degree of hydroxyl and high substitution, applied in the field of preparation of functionalized graphene, can solve the problem of low degree of substitution of surface active groups
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Step 1: Preparation of Graphene Oxide
[0022] Graphene oxide (GO) was prepared by the improved Hummers method. Take 5 g of expanded graphite, add 115 ml of concentrated sulfuric acid to it, place it in an ice bath at 0 °C, and then slowly add 15 g of KMnO 4 . Then the mixture was stirred and reacted at 35 °C for 12 h. After the reaction was completed, 230 ml of deionized water was slowly added to the flask, and the temperature was raised to 98 °C and kept at this temperature for 15 min. Then add 700 ml of deionized water therein for dilution to terminate the reaction. After dilution, 30% concentration of H 2 o 2 (30 ml) was added to the above mixture, and the solution quickly turned bright yellow with bubbles. The mixture was filtered, rinsed with 5% dilute hydrochloric acid aqueous solution (500 ml) to remove metal ions in the graphene aqueous solution, and then washed with 2,000 ml of deionized water to remove excess acid, and the obtained sample was placed in an...
Embodiment 2
[0028] Step 1: Preparation of Graphene Oxide
[0029] Graphene oxide (GO) was prepared by the improved Hummers method. Take 5 g of expanded graphite, add 115 ml of concentrated sulfuric acid to it, place it in an ice bath at 0 °C, and then slowly add 15 g of KMnO 4 . Then the mixture was stirred and reacted at 35 °C for 12 h. After the reaction was completed, 230 ml of deionized water was slowly added to the flask, and the temperature was raised to 98 °C and kept at this temperature for 15 min. Then add 700 ml of deionized water therein for dilution to terminate the reaction. After dilution, 30% concentration of H 2 o 2(30 ml) was added to the above mixture, and the solution quickly turned bright yellow with bubbles. The mixture was filtered, rinsed with 5% dilute hydrochloric acid aqueous solution (500 ml) to remove metal ions in the graphene aqueous solution, and then washed with 2,000 ml of deionized water to remove excess acid, and the obtained sample was placed in an ...
Embodiment 3
[0035] Step 1: Preparation of Graphene Oxide
[0036] Graphene oxide (GO) was prepared by the improved Hummers method. Take 5 g of expanded graphite, add 115 ml of concentrated sulfuric acid to it, place it in an ice bath at 0 °C, and then slowly add 15 g of KMnO 4 . Then the mixture was stirred and reacted at 35 °C for 12 h. After the reaction was completed, 230 ml of deionized water was slowly added to the flask, and the temperature was raised to 98 °C and kept at this temperature for 15 min. Then add 700 ml of deionized water therein for dilution to terminate the reaction. After dilution, 30% concentration of H 2 o 2 (30 ml) was added to the above mixture, and the solution quickly turned bright yellow with bubbles. The mixture was filtered, rinsed with 5% dilute hydrochloric acid aqueous solution (500 ml) to remove metal ions in the graphene aqueous solution, and then washed with 2,000 ml of deionized water to remove excess acid, and the obtained sample was placed in an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com