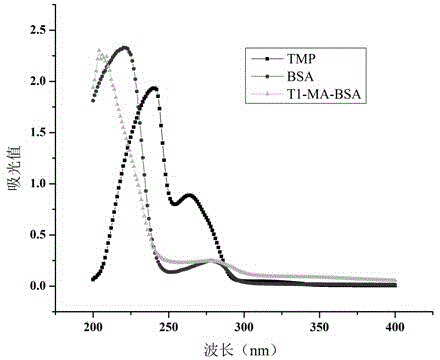
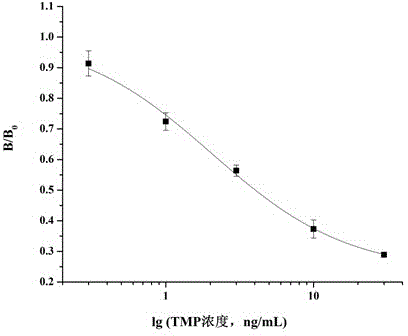
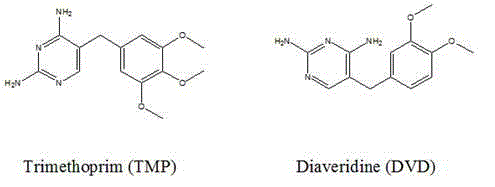
Method for preparing complete antigen from trimethoprim semiantigen compound T1 and use of complete antigen
A technology of trimethoprim and complete antigen, applied in the field of biochemical industry, to achieve the effect of high specificity and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1 Preparation of Trimethoprim Hapten T1
[0027] Add 10mL of pyridine, 2g of succinic anhydride, and 5mg of 4-dimethylaminopyridine in sequence to a 100mL three-necked flask, and heat in a water bath at 60°C with magnetic stirring until the solution is clear. Then 2.9 g of trimethoprim was dissolved in 10 mL of pyridine and added dropwise to the three-necked flask, and the stirring reaction was continued for 3 days. TLC was used to monitor the progress of the reaction using methanol: chloroform with a volume ratio of 1:5 as the developing solvent. Distill at 50°C for 10 minutes under reduced pressure at 0.090 MPa to evaporate all the solvent to obtain a pale yellow oil. Add dropwise mass volume ratio 10% NaHCO 3 The solution was no longer turbid, filtered and the supernatant was recrystallized under acidic conditions of pH 5.0 to precipitate a white solid, centrifuged at 6000 r / min for 10 min, and the filter cake was dried at 50°C to obtain the trimethoprim ha...
Embodiment 2
[0028] Example 2 Preparation of Trimethoprim Complete Antigen
[0029] Take 8 mg of trimethoprim hapten T1, dissolve it in 1 mL of DMF, and mix it with tri-n-butylamine and isobutyl chloroformate (the molar ratio of T1: tri-n-butylamine: isobutyl chloroformate is 1: 1.2: 1.2) 0 The reaction was carried out at ℃ for 1 h to obtain the activated trimethoprim hapten compound T1 solution. Take BSA / OVA (the molar ratio of hapten T1 to BSA and OVA is 100:1), dissolve it in 0.01M pH6.5 phosphate buffer, pre-cool at 0°C for 30 minutes, and the dissolved protein concentration is greater than 3mg / mL , and the volume ratio of phosphate buffer saline to DMF was 5:1 to obtain a protein solution. At 0°C, the activated hapten T1 solution was slowly added dropwise to the protein solution, reacted at 0°C for 2 hours, and then reacted at room temperature for 12 hours. Dialyze with PBS buffer for 3 days, during which the water was changed 8 times to obtain the complete trimethoprim antigen (T1-...
Embodiment 3
[0030] Example 3 Preparation of Trimethoprim Antiserum
[0031] Using the antigen (T1-MA-BSA) prepared in Example 2 as the immunogen, 8-week-old female BALB / C mice were selected as immunized animals, and Freund's adjuvant was used for immunization, and 5 mice were immunized. The Freund's adjuvant immunization method is as follows: firstly, take an appropriate amount of immunogen and mix it with an equal volume of Freund's complete adjuvant; Inject immunization at multiple points, and boost immunization every 3 weeks.
PUM
| Property | Measurement | Unit |
|---|---|---|
| reaction rate constant | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com