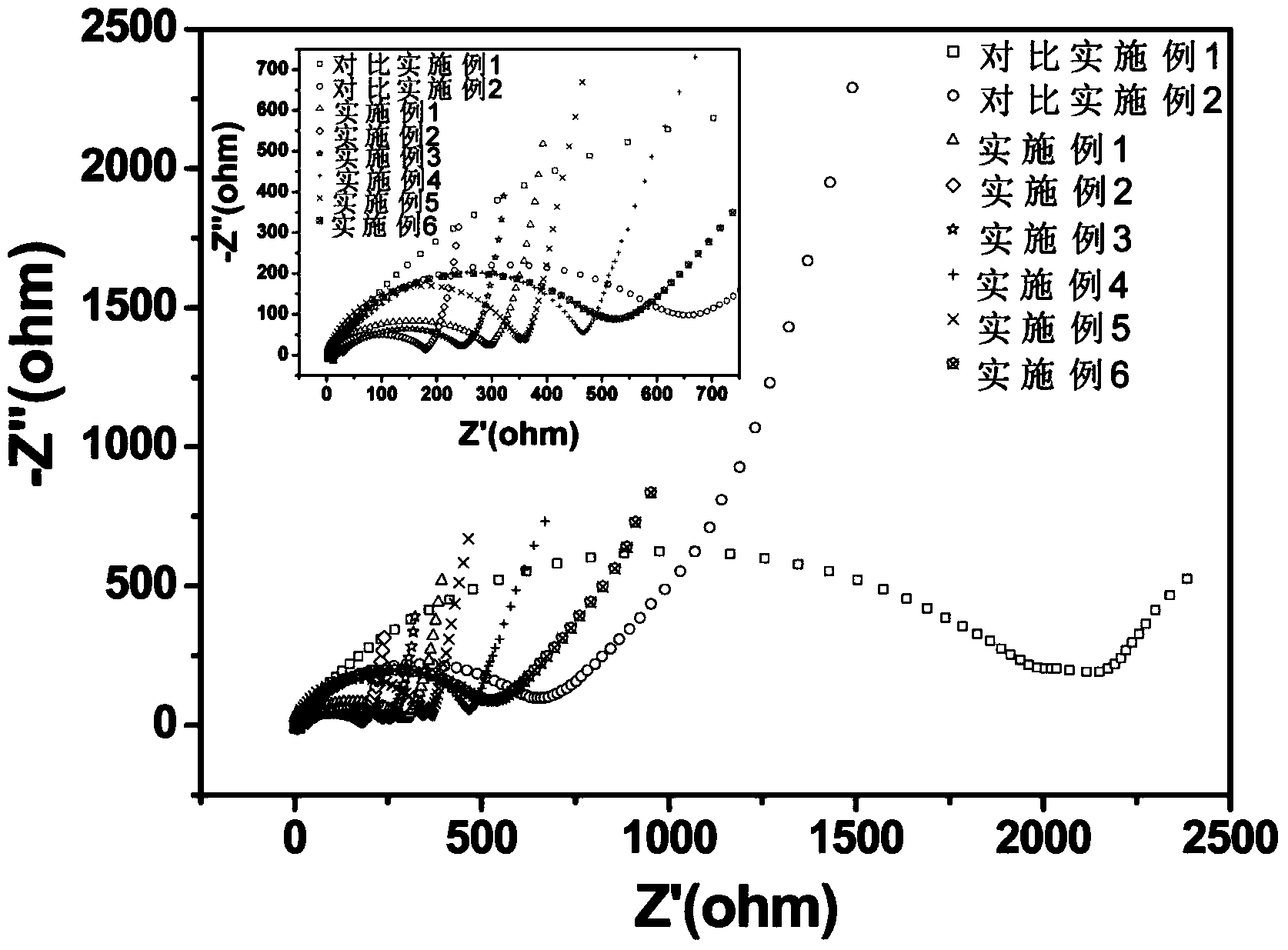
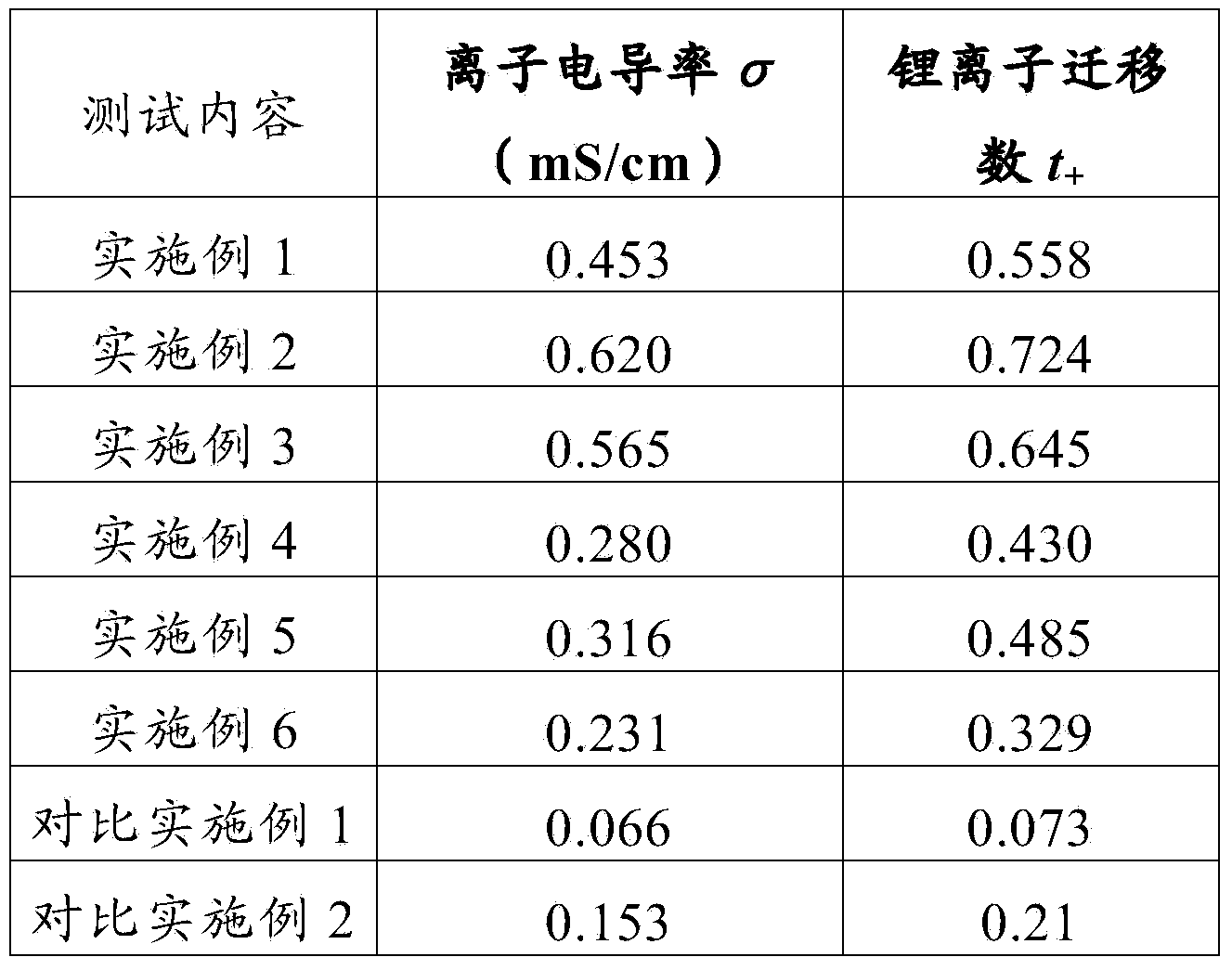
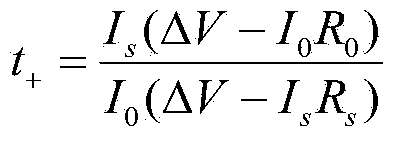Polymer electrolyte membrane and preparation method thereof
A technology of electrolyte membrane and polymer, which is applied in the field of electrolyte membrane for lithium-ion batteries, can solve the problems of poor ion conductivity of particles, affect the performance of electrolyte membrane, and poor dispersion, so as to improve the conductivity of lithium ions and the migration number of lithium ions , good elasticity and processing performance, good mechanical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Get 2.48g lithium trifluoromethanesulfonimide (LiTFSI, LiN(SO 2 CF 3 ) 2 )and 1 . 5g fast ion conductor (Li 1.3 al 0.3 Ti 1.7 (PO 4 ) 3 ) doped TiO 2 Inorganic filler powder (200-500nm) was added to 100 g of 1-methyl-2-pyrrolidone solution of MEEEP with a mass fraction of 10%, stirred and dispersed at 60°C for 20 hours, and cast on a polypropylene non-woven membrane The polymer electrolyte membrane was obtained after vacuum drying at 70°C for 48 hours.
Embodiment 2
[0020] Get 1.24g lithium trifluoromethanesulfonylimide (LiTFSI, LiN(SO 2 CF 3 ) 2 ), 0.84g lithium bisoxalate borate (LiBOB, LiB(C 2 o 4 ) 2 ) and 1.0g fast ion conductor (Li 1.3 al 0.3 Ti 1.7 (PO 4 ) 3 ) doped TiO 2 Inorganic filler powder (200-500nm) was added to 160 grams of MEEEP dimethylformamide solution with a mass fraction of 5%, stirred and dispersed at 40°C for 12 hours, and cast on a polypropylene non-woven membrane to form a film. The polymer electrolyte membrane was obtained after vacuum drying at 70° C. for 48 hours.
Embodiment 3
[0022] Get 1.34g lithium bisoxalate borate (LiBOB, LiB(C 2 o 4 ) 2 ) and 1.92g fast ion conductor (Li 1.3 al 0.3 Ti 1.7 (PO 4 ) 3 ) doped SiO 2 Inorganic filler powder (80 ~ 200nm) was added to 120 grams of dimethyl sulfoxide solution with a mass fraction of 8% MEEEP, stirred and dispersed at 50°C for 16 hours, and cast on a polypropylene non-woven diaphragm to form a film at 70 The polymer electrolyte membrane was obtained after vacuum drying at ℃ for 36 hours.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com