Production method for preparing ferric hydroxide by utilizing ferrite-containing waste water
A technology of ferric hydroxide and production method, which is applied in the direction of iron oxide/ferric hydroxide, chemical instruments and methods, multi-stage water/sewage treatment, etc., and can solve the problems of large consumption, large amount of mud production, secondary pollution, etc. , to achieve the effect of ensuring economic benefits, high oxidation rate and strong oxidation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
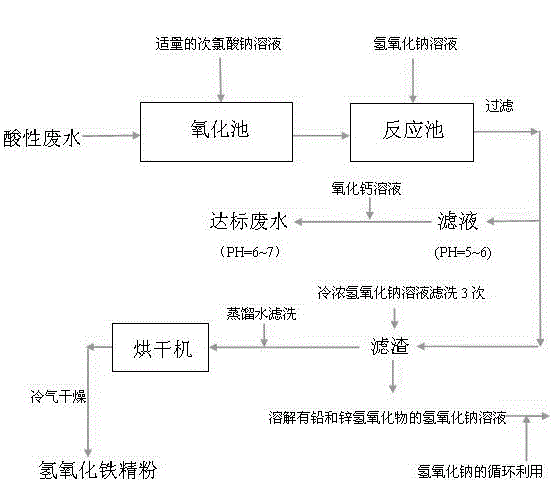
[0019] In a steel wire rope factory, the initial pH value of the chemical wastewater discharged during the production process is less than 1, Fe 2+ The content is 90~95g / L. Send the wastewater into the oxidation tank, and add active Cl to each ton of wastewater while stirring - ≥5.2% (mass percentage), 7.0~8.0% (mass percentage) of free alkali NaClO solution 814L (the concentration of the substance is 0.7334mol / L), so that all the ferrous ions in the wastewater are converted into ferric ions ;Introduce the fully oxidized wastewater into the reaction tank, and add sodium hydroxide solution to the wastewater pH=5~6 while stirring, at this time, hydroxide ions, ferric ions, lead ions, and zinc ions are combined to generate hydrogen Iron oxide flocculent precipitation, lead hydroxide, zinc hydroxide precipitation. Filter the solution containing ferric hydroxide, lead hydroxide, and zinc hydroxide precipitation to obtain filter residue and filtrate; take the filter residue in a f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com