Preparation method for 5-chlorine-2-hydroxyl-3-nitroacetophenone
A technology of nitroacetophenone and hydroxyacetophenone, which is applied in the field of preparation of pharmaceutical intermediates, can solve the problems of large solvent consumption, safety accidents, environmental pollution, and high cost, and achieve simple and convenient follow-up processing, reduce production costs, The effect of a short reaction cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
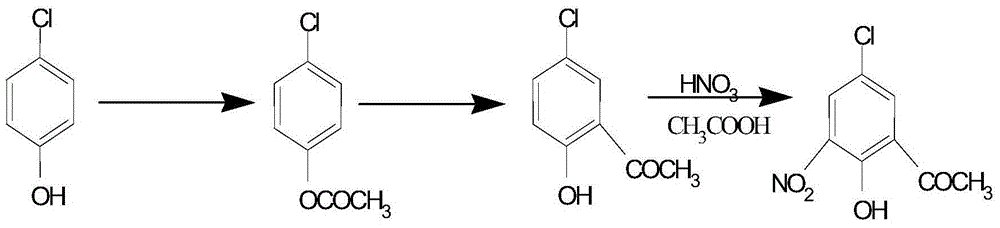
[0029] Add 77.2g (0.6mol) of p-chlorophenol and 73.4g (0.72mol) of acetic anhydride into a 500mL three-neck flask, add 5g of concentrated sulfuric acid dropwise into the flask, stir, react at 110°C for 1.5h, and carry out vacuum distillation. The fraction is glacial acetic acid, and the fraction at 90°C is collected to obtain 100 g of p-chlorophenol acetate with a purity of 98.2%;
[0030] Add 34.2g (0.2mol) of p-chlorophenol acetate into a 500mL three-neck flask, slowly add 79.5g (about 0.6mol) of anhydrous aluminum trichloride to the flask and stir for 1 hour at 130°C. Slowly add 200 mL of water into the flask, stir for 0.5 h, filter, heat the filter cake with methanol to dissolve, decolorize with activated carbon, and recrystallize to obtain 30.8 g of 5-chloro-2-hydroxyacetophenone with a purity of 99.68%.
[0031] Add 43g (0.25mol) of 5-chloro-2-hydroxyacetophenone into a 500mL three-necked flask, slowly add 200mL glacial acetic acid to the flask, stir, and slowly dissolve...
Embodiment 2
[0033] Add 77.2g (0.6mol) of p-chlorophenol and 73.4g (0.72mol) of acetic anhydride into a 500mL three-neck flask, add 5g of potassium carbonate into the flask, stir, react at 100°C for 1.5h, filter, and conduct vacuum distillation , the previous fraction was glacial acetic acid, and the fraction collected at 90°C was 98.7g of p-chlorophenol acetate, with a purity of 98.7%;
[0034] Add 34.2g (0.2mol) of p-chlorophenol acetate into a 500mL three-necked flask, slowly add 79.5g (about 0.6mol) of anhydrous aluminum trichloride to the flask, stir and react at 120°C for 1h, Slowly add 200 mL of water into the flask, stir for 0.5 h, filter, heat the filter cake with methanol to dissolve, decolorize with activated carbon, and recrystallize to obtain 30.5 g of 5-chloro-2-hydroxyacetophenone with a purity of 99.68%.
[0035] Add 43g (0.25mol) of 5-chloro-2-hydroxyacetophenone into a 500mL three-necked flask, slowly add 200mL of glacial acetic acid into the flask, stir, and slowly disso...
Embodiment 3
[0037] Add 77.2g (0.6mol) of p-chlorophenol and 73.4g (0.72mol) of acetic anhydride into a 500mL three-necked flask, add 5g of triethylamine into the flask, stir, react at 120°C for 1.5h, filter, and decompress Distillation, the front fraction is glacial acetic acid, and the fraction at 90°C is collected to obtain 96.7 g of p-chlorophenol acetate with a purity of 98.3%;
[0038] Add 34.2g (0.2mol) of p-chlorophenol acetate into a 500mL three-necked flask, slowly add 79.5g (about 0.6mol) of anhydrous aluminum trichloride to the flask, stir and react at 140°C for 1h, Slowly add 200 mL of water into the flask, stir for 0.5 h, filter, heat the filter cake with methanol to dissolve, decolorize with activated carbon, and recrystallize to obtain 31 g of 5-chloro-2-hydroxyacetophenone with a purity of 99.58%.
[0039] Add 43g (0.25mol) of 5-chloro-2-hydroxyacetophenone into a 500mL three-necked flask, slowly add 200mL glacial acetic acid into the flask, stir, and slowly dissolve, then...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com