Preparation methods of optically pure rabeprazole and sodium salt thereof
A technology of rabeprazole sodium and rabeprazole, which is applied in the field of preparation and purification of high-purity chiral rabeprazole and its sodium salt, can solve the problems of difficulty in determining the amount of NaOH, lower yield, etc., and achieve the benefit of Effects of chemical purity, low cost, and improved optical purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
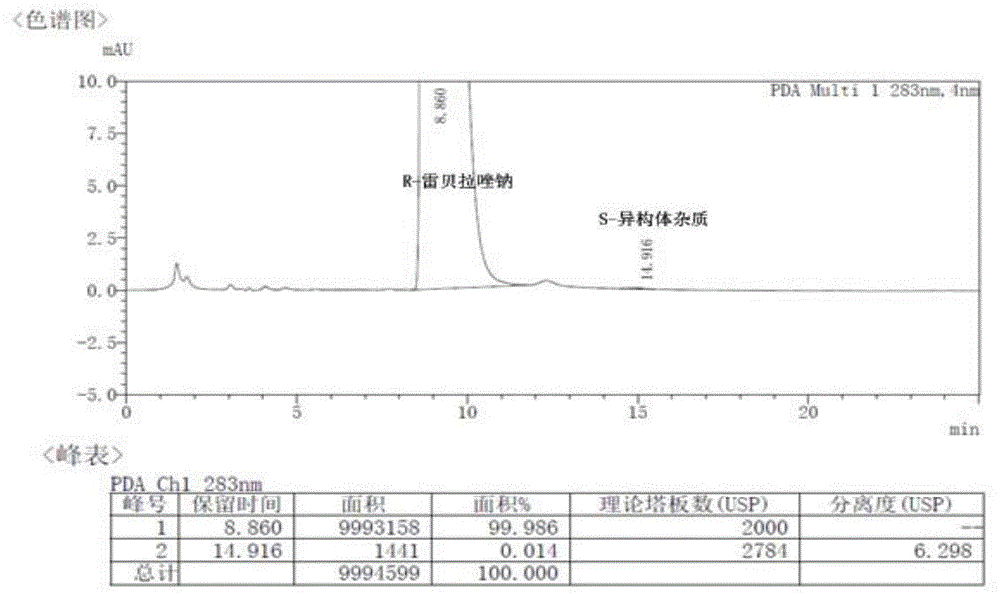
[0033] Embodiment 1: the purification of R-(+)-rabeprazole
[0034] Add 9g of crude R-(+)-rabeprazole with 95% purity and 100mL of toluene, stir to dissolve, and extract the mixture with 12.5% ammonia water three times (50mL×3), and combine the aqueous phases. Take the aqueous phase, add 80 mL of methyl isobutyl ketone, and adjust the pH to 9.0 with 80% acetic acid. The liquid was separated, and the aqueous phase was extracted twice with methyl isobutyl ketone (50mL×2). Combine the organic phases with pH 10.5 K 3 PO 4 -K 2 HPO 4 Wash once with 25 mL of buffer solution. Take the organic layer, lower the temperature, let stand, and precipitate the solid. Suction filtration, washing with methyl isobutyl ketone, and drying gave 7.5 g of a white solid with a chemical purity of 99.93% (achiral analysis) and an ee value of 99.98% (chiral analysis).
Embodiment 2
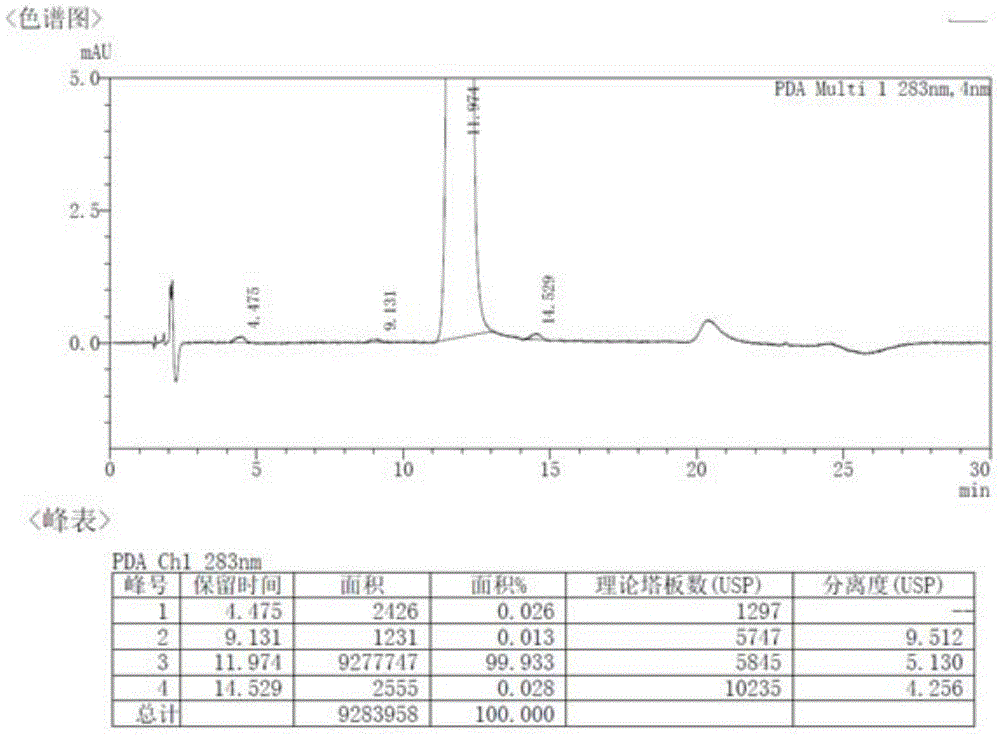
[0035] Embodiment 2: the purification of S-(-)-rabeprazole
[0036] Add 9 g of crude S-(-)-rabeprazole with 70% purity and 100 mL of toluene, stir to dissolve, and extract the mixture three times with 12.5% ammonia water (50 mL×3), and combine the aqueous phases. Take the water phase, add 80 mL of methyl isobutyl ketone, and adjust the pH to 9.5 with 80% acetic acid. The liquid was separated, and the aqueous phase was extracted twice with methyl isobutyl ketone (50mL×2). Combine the organic phases and wash with Na at pH 10.5 2 CO 3 -NaHCO 3 Wash once with 25 mL of buffer solution. Take the organic layer, lower the temperature, let stand, and precipitate the solid. Suction filtration, washing with methyl isobutyl ketone, and drying gave 5.9 g of a white solid with a chemical purity of 99.91% (achiral analysis) and an ee value of 99.95% (chiral analysis).
Embodiment 3
[0037] Embodiment 3: the preparation of R-(+)-rabeprazole
[0038] Add 2-[4-(3-methoxypropoxy-3-methyl-2-pyridyl)methylthio]-1H-benzimidazole 20.0g, toluene 200mL, L-(+)-tartaric acid di Ethyl ester 6.2mL, titanium tetraisopropoxide 5.2mL, water 0.14mL. After the addition was complete, the reaction was stirred at 54°C for 1h. Cool down to below 20°C, add 3.1 mL of N,N-diisopropylethylamine and 10.2 mL of cumene hydroperoxide. After addition, the reaction was stirred at 20°C for 2h. After the reaction was completed, the reaction solution was extracted three times with 12.5% ammonia water (50 mL×3), and the aqueous phase was combined. Take the water phase, add 120 mL of methyl isobutyl ketone, and adjust the pH to 9.5 with 80% acetic acid. The liquid was separated, and the aqueous phase was extracted twice with methyl isobutyl ketone (50mL×2). Combine the organic phases, lower the temperature, let stand, and precipitate the solid. Suction filtration, washing with methyl ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical purity | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com