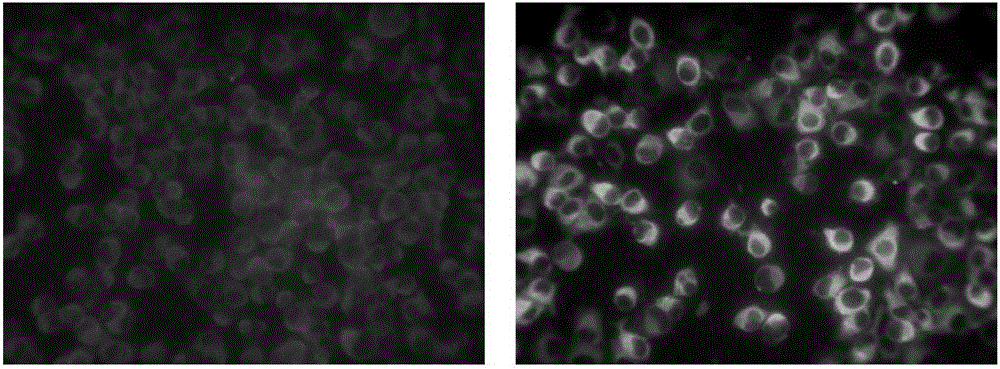
Monoclonal antibody and kit for detecting tick-borne encephalitis virus
A monoclonal antibody and encephalitis virus technology, applied in the direction of resistance to vector-borne diseases, measuring devices, instruments, etc., can solve the problems of high false positives and up to 10 months
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0042] The preparation method of the virus antigen sheet used in following embodiment is as follows:
[0043] 1) Stay 25cm 2 When the BHK21 cells (baby hamster kidney passage cells) in the square bottle grow to about 80%, infect the cells with 1ml of the virus strain, after 1 hour, suck out the adsorption solution, and add the cell maintenance solution (DMEM culture solution containing 2% FBS) , placed in a 37°C incubator for cultivation;
[0044] 2) When 25%-50% of the cells just appear cytopathic, digest the cells according to the method of cell passage, suspend the cells with an appropriate volume of cell culture medium (DMEM medium containing 10% FBS), add 40ul to each antigen well Cell suspension, put into the incubator and continue to cultivate for 8 hours;
[0045]3) Take a container containing PBS solution, slowly put the antigen sheet cultured for 8 hours in the previous step, soak for 1 min, take out the antigen sheet and put it on the workbench to dry;
[0046] 4...
Embodiment 1
[0048] Example 1. Screening and preparation of monoclonal antibodies for detection of tick-borne encephalitis virus
[0049] 1. Preparation and purification of immunogen-
[0050] Tick-borne encephalitis virus strain Senzhang was used to inoculate BHK-21 cells at 37°C, 5% CO 2 Cultivate in an incubator, and when more than 75% of the cells have lesions, centrifuge the culture solution at 4°C and 10,000 rpm for 30 minutes, and take the supernatant; use β-propiolactone (purchased from Seebio, Cat. No. 168-21011) to inactivate the virus, Obtain the immunogen and store it in a -70°C refrigerator for later use.
[0051] 2. Animal immunity
[0052] 1. Measure the total protein content of the immunogen obtained in step 1, and adjust the protein concentration to 0.5 mg / mL with antibody diluent.
[0053] 2. Basic immunization: emulsify the 0.5 mg / mL immunogen solution obtained in step 1 with an equal volume of Freund's complete adjuvant, and then subcutaneously inject 12-week-old fem...
Embodiment 2
[0072] Example 2, Screening and Preparation of Monoclonal Antibodies for Detection of Flaviviruses
[0073] 1. Preparation and purification of immunogen
[0074] Same as Step 1 in Example 1.
[0075] 2. Animal immunity
[0076] Same as Step 2 in Example 1.
[0077] 3. Cell fusion and cloning
[0078] From the second booster immunization, on the 3rd day after each immunization, blood was collected from the mouse orbit to determine the antibody titer, and the mouse with the best serum titer was selected, and splenocytes were taken to fuse with SP2 / 0 myeloma cells; Screen hybridoma cell lines that secrete monoclonal antibodies by limiting dilution method; use indirect non-competitive ELISA to screen out the monoclonal that stably secretes the monoclonal antibody with the highest titer (titer 1:10240) against the immunogen obtained in step 1 The hybridoma cell line, named 2A10, was preserved on August 19, 2013 in the General Microbiology Center of China Committee for Culture C...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com